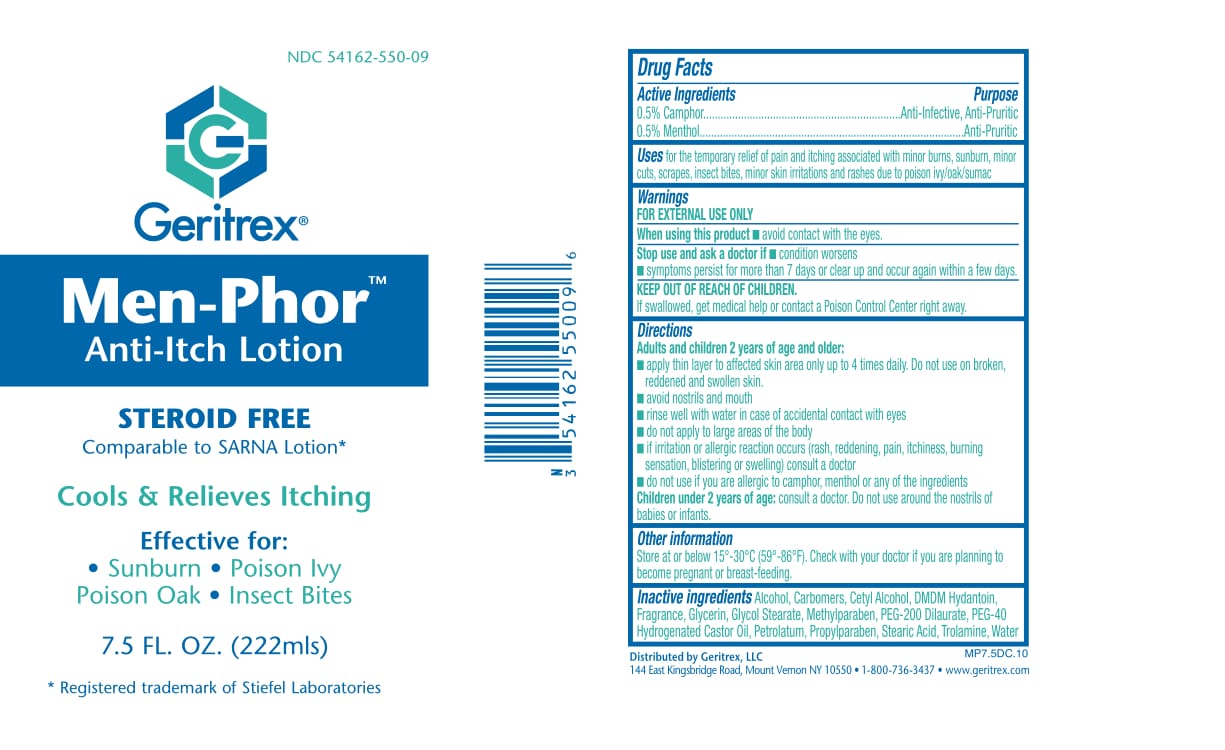
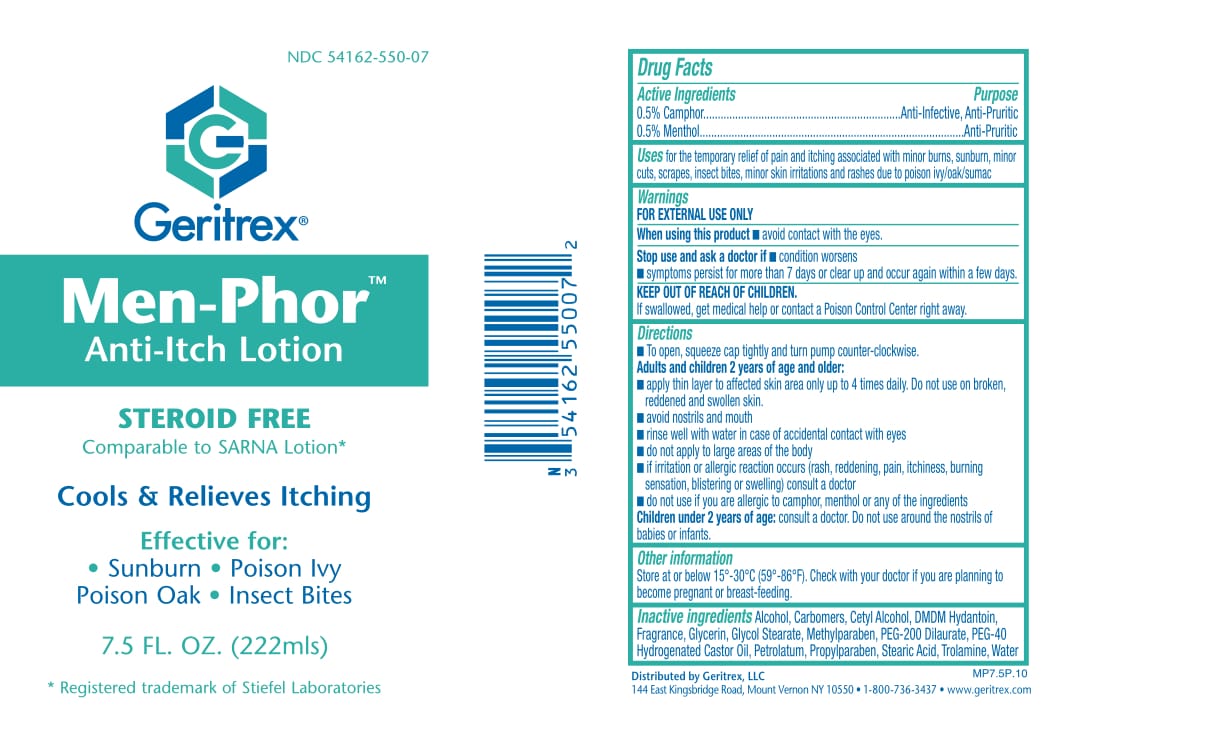
MEN-PHOR- menthol, camphor lotion
Geritrex LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Active Ingredients Purpose
0.5% Camphor . . . .Anti-Infective, Anti-Pruritic
0.5% Menthol . . . .Anti-Pruritic
Uses
To provide temporary relief for dry
itching skin, sunburn, insect bites and pruritus.
Directions
• Apply two or three times daily, or as directed
by your physician.
Warnings
For External Use Only
• Avoid contact with eyes. If contact occurs,
rinse eyes thoroughly with water.
• If improvement does not occur, or condition
worsens after regular use as directed,
discontinue use and consult a physician.
• Do not use on children under two years of age.
• Do not apply under compresses or bandages
Other Ingredient
Alcohol, Carbomers, Cetyl Alcohol, DMDM Hydantoin, Fragrance, Glycerin, Glycol Stearate, Methylparaben, PEG-200 Dilaurate, PEG-40 Hydrogenated Castor Oil, Petrolatum, Propylparaben, Stearic Acid, Trolamine, Water
Keep out of reach of children
Apply two or three times daily.