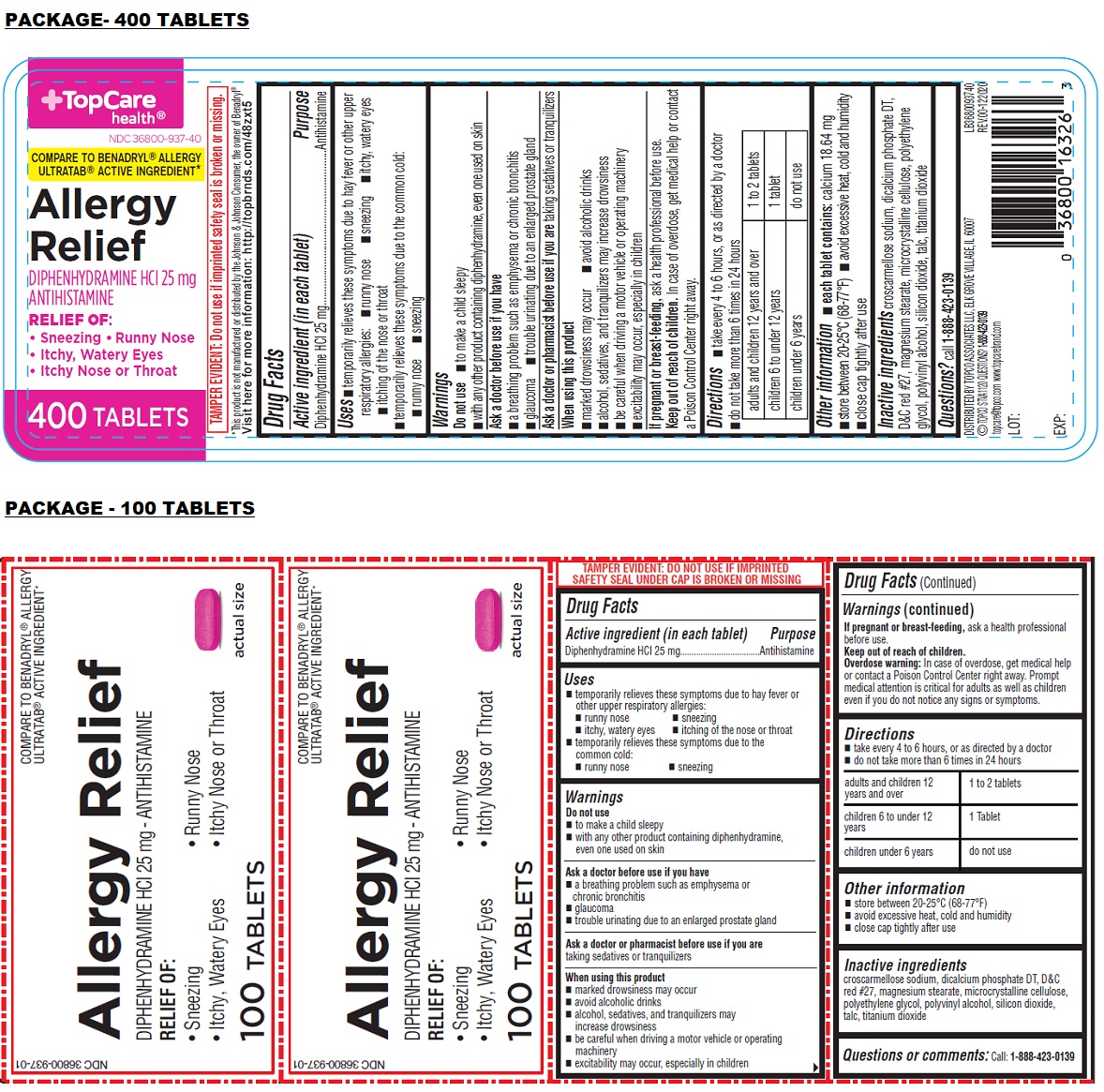
Uses
• temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
• runny nose • sneezing • itchy, watery eyes • itching of the nose or throat
• temporarily relieves these symptoms due to the common cold:
• runny nose • sneezing
Warnings
Do not use
• to make a child sleepy
• with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
• a breathing problem such as emphysema or chronic bronchitis
• glaucoma
• trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
• marked drowsiness may occur
• avoid alcoholic drinks
• alcohol, sedatives, and tranquilizers may increase drowsiness
• be careful when driving a motor vehicle or operating machinery
• excitability may occur, especially in children
If pregnant or breast-feeding, ask a health professional before use.
Directions
• take every 4 to 6 hours, or as directed by a doctor
• do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 tablets |
| children 6 to under 12 years | 1 tablet |
| children under 6 years | do not use |
Other information
• store between 20-25°C (68-77°F)
• avoid excessive heat, cold and humidity
• close cap tightly after use
Inactive ingredients
croscarmellose sodium, dicalcium phosphate DT, D&C red #27, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, talc, titanium dioxide