FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
RELYVRIO is indicated for the treatment of amyotrophic lateral sclerosis (ALS) in adults.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended initial dosage of RELYVRIO for oral suspension is 1 packet (3 g sodium phenylbutyrate and 1 g taurursodiol) daily for the first 3 weeks. After 3 weeks, increase to the maintenance dosage of 1 packet twice daily.
2.2 Preparation and Administration Information
Empty contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously. Take orally or administer via feeding tube within 1 hour of preparation. The reconstituted suspension may be stored for up to 1 hour at room temperature. Discard any unused RELYVRIO reconstituted suspension after 1 hour.
Administer RELYVRIO before a snack or meal.
3 DOSAGE FORMS AND STRENGTHS
For oral suspension: white to yellow powder provided in single-dose packets each containing 3 g sodium phenylbutyrate and 1 g taurursodiol.
5 WARNINGS AND PRECAUTIONS
5.1 Risk in Patients with Enterohepatic Circulation Disorders, Pancreatic Disorders, or Intestinal Disorders
RELYVRIO contains taurursodiol, which is a bile acid. In patients with disorders that interfere with bile acid circulation, there may be an increased risk for worsening diarrhea, and patients should be monitored appropriately for this adverse reaction. Pancreatic insufficiency, intestinal malabsorption, or intestinal diseases that may alter the concentration of bile acids, may also lead to decreased absorption of either of the components of RELYVRIO. Because different enterohepatic circulation, pancreatic, and intestinal disorders have varying degrees of severity, consider consulting with a specialist. Patients with disorders of enterohepatic circulation (e.g., biliary infection, active cholecystitis, etc.), severe pancreatic disorders (e.g., pancreatitis), and intestinal disorders that may alter concentrations of bile acids (e.g., ileal resection, regional ileitis, etc.) were excluded from the study, therefore there is no clinical experience in these conditions.
5.2 Use in Patients Sensitive to High Sodium Intake
RELYVRIO has a high salt content. Each initial daily dosage of one packet contains 464 mg of sodium; each maintenance dosage of two packets daily contains 928 mg of sodium. In patients sensitive to salt intake (e.g., those with heart failure, hypertension, or renal impairment), consider the amount of daily sodium intake in each dose of RELYVRIO and monitor appropriately.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk in Patients with Enterohepatic Circulation Disorders, Pancreatic Disorders, or Intestinal Disorders [see Warnings and Precautions (5.1)]
- Use in Patients Sensitive to High Sodium Intake [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of RELYVRIO was evaluated in Study 1 which enrolled 137 adult patients with ALS randomized (2:1) to RELYVRIO (n = 89) or placebo (n = 48) for 24 weeks.
In Study 1, there were 5 (6%) RELYVRIO-treated patients and 2 (4%) placebo patients who died during the 24-week study. The deaths appeared to be related to ALS disease progression.
The most common adverse reactions (at least 15% and at least 5% greater than placebo) with RELYVRIO were diarrhea, abdominal pain, nausea, and upper respiratory tract infection. Gastrointestinal-related adverse reactions occurred throughout the study but were more frequent during the first 3 weeks of treatment.
Table 1 lists the common adverse reactions that occurred in more than 5% of patients taking RELYVRIO and were at least 5% greater than in patients taking placebo in Study 1.
| Adverse Reaction |
RELYVRIO
|
Placebo |
| Diarrhea* | 25 | 19 |
| Abdominal pain* | 21 | 13 |
| Nausea | 18 | 13 |
| Upper respiratory tract infection*
| 18 | 10 |
| Fatigue*
| 12 | 6 |
| Salivary hypersecretion | 11 | 2 |
| Dizziness | 10 | 4 |
|
*Adverse reaction is composed of several similar terms. |
||
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect RELYVRIO
Bile Acid Sequestering Agents
Bile acid sequestering agents (e.g., cholestyramine, colestipol, colesevelam) may interfere with the absorption of bile acids such as taurursodiol. Avoid use of bile acid sequestering agents with RELYVRIO and consider alternative cholesterol lowering agents.
Inhibitors of Bile Acid Transporters
Concomitant medications that inhibit canalicular membrane bile acid transporters such as the bile salt export pump (BSEP) may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. Avoid use of strong inhibitors of BSEP with RELYVRIO. If concomitant use of a strong inhibitor of BSEP (e.g., cyclosporine) is deemed necessary, caution should be exercised and monitoring of serum transaminases and bilirubin is recommended.
Aluminum-based Antacids
Aluminum-based antacids have been shown to adsorb bile acids in vitro and may interfere with the absorption of taurursodiol. Avoid use of aluminum-based antacids with RELYVRIO and consider other acid lowering agents.
Probenicid
Avoid use of probenecid with RELYVRIO as it may affect renal excretion of sodium phenylbutyrate metabolites.
HDAC Inhibitors
Phenylbutyrate is a pan-histone deacetylase (HDAC) inhibitor. Avoid use of other HDAC inhibitors with RELYVRIO.
Inhibitors of Transports OATP1B3
In vitro studies showed that RELYVRIO is a substrate of OATP1B3. Avoid use of inhibitors of OATP1B3 with RELYVRIO.
7.2 Potential for RELYVRIO to Affect Other Drugs
OAT1 Substrates
Plasma concentrations of OAT1 substrates may be increased if given concomitantly with RELYVRIO. Avoid use of OAT1 substrates for which a small change in substrate plasma concentration may lead to serious toxicities or loss of efficacy with RELYVRIO.
P-glycoprotein (P-gP) and Breast Cancer Resistance Protein (BCRP) Substrates
RELYVRIO has been shown to inhibit P-gP and BCRP in vitro. Plasma concentrations of P-gP and BCRP substrates may be increased if given concomitantly with RELYVRIO. Avoid the concomitant use of P-gP and BCRP substrates for which a small change in substrate plasma concentration may lead to serious toxicities or loss of efficacy with RELYVRIO.
Drugs that are substrates of CYP2C8, CYP1A2, CYP2B6, and CYP3A4isoenzymes
RELYVRIO inhibits CYP2C8 and CYP2B6 isoenzymes in vitro. RELYVRIO induces CYP1A2, CYP2B6, and CYP3A4 in vitro. Plasma concentrations of substrates for these enzymes may be changed if given concomitantly with RELYVRIO. Avoid use of drugs that are substrates of these CYP P450 isoenzymes in which a small change in substrate plasma concentration may lead to serious toxicities or loss of efficacy.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on RELYVRIO use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal studies, administration of sodium phenylbutyrate and taurursodiol to rats throughout pregnancy and lactation resulted in increased offspring mortality at all doses tested, which were less than or similar to the clinical doses.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day, containing sodium phenylbutyrate and taurursodiol in a 3:1 ratio) to pregnant mice during the period of organogenesis was not associated with any adverse effects. At the highest dose of the combination of sodium phenylbutyrate and taurursodiol tested, the doses of sodium phenylbutyrate and taurursodiol were similar to the maximum recommended dose (6 g sodium phenylbutyrate and 2 g taurursodiol) in humans (MRHDs), based on body surface area (mg/m2).
Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day) to pregnant rats during the period of organogenesis was not associated with any adverse effects. At the highest dose of the combination of sodium phenylbutyrate and taurursodiol tested, the doses of sodium phenylbutyrate and taurursodiol were approximately 2-fold the MRHDs, based on mg/m2.
Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day) to rats throughout pregnancy and lactation resulted in increases in stillbirth at all doses and pup deaths at the highest dose tested. A no effect dose for adverse developmental effects in rats was not identified. At the lowest dose of the combination of sodium phenylbutyrate and taurursodiol tested, the doses of sodium phenylbutyrate and taurursodiol were less than the MRHDs, based on mg/m2.
8.2 Lactation
Risk Summary
There are no data on the presence of sodium phenylbutyrate or taurursodiol in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RELYVRIO and any potential adverse effects on the breastfed child from RELYVRIO or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of RELYVRIO in pediatric patients have not been established.
8.5 Geriatric Use
Of the 89 patients with ALS who received RELYVRIO in Study 1, 25 patients (28%) were 65 years of age or older, while 4 patients (4.5%) were 75 years of age and older with the oldest patient being 79 years old.
No overall differences in safety or effectiveness were observed between those patients 65 years of age and older and those <65 years of age. Although differences in responses between the elderly and younger patients were not identified, greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dose adjustment is needed for patients with mild renal impairment. Avoid use in patients with moderate or severe renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is needed for patients with mild hepatic impairment. Avoid use in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
RELYVRIO contains two active ingredients: sodium phenylbutyrate and taurursodiol.
The chemical designation for phenylbutyrate is 4-phenyl butyric acid sodium salt. Its molecular formula is C10H11NaO2, and its molecular weight is 186.2. The sodium phenylbutyrate chemical structure is:
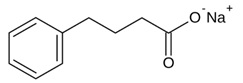
Sodium phenylbutyrate is a white or yellow powder which decomposes at about 220°C. It is freely soluble in water and methanol; sparingly soluble in ethanol; and practically insoluble in methylene chloride, acetone, and diethyl ether.
Taurursodiol, also known as tauroursdeoxycholic acid, is an ambiphilic bile acid and is the taurine conjugate of ursodiol, also known as ursodeoxycholic acid. The chemical name of taurursodiol is 2-[(3α, 7β-dihydroxy-24-oxo-5β-cholan-24-yl) amino] ethane sulfonic acid, dihydrate. The molecular formula is C26H45NO6S . 2H2O and the molecular weight is 535.74 (dihydrate).
The taurursodiol chemical structure is:
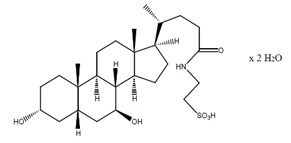
Taurursodiol is a white microcrystalline powder, practically odorless, with a bitter taste. It is freely soluble in ethyl alcohol, very slightly soluble in acetone and dioxane, sparingly soluble in water, and practically insoluble in ether and ethyl acetate.
RELYVRIO is a white to yellow powder for oral suspension that consists of fine to large granules. RELYVRIO is supplied in a packet containing 3 g sodium phenylbutyrate (equivalent to 2630 mg phenylbutyrate) and 1 g taurursodiol. Each packet contains 464 mg of sodium and also contains the following inactive ingredients: acacia, dextrates, dibasic sodium phosphate, maltodextrin, medium-chain triglycerides, mixed berry flavoring, other flavoring ingredients, silicon dioxide, sodium stearyl fumarate, sorbitol, and sucralose.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which RELYVRIO exerts its therapeutic effects in patients with ALS is unknown.
12.2 Pharmacodynamics
Cardiac Electrophysiology
At the maximum recommended dose, RELYVRIO does not cause large mean increases (>20 ms) in the QT interval.
12.3 Pharmacokinetics
Absorption
Following oral administration of a single dose of RELYVRIO in healthy subjects under fasting conditions, sodium phenylbutyrate reaches a median Tmax of 0.5 hour. Taurursodiol reaches a median Tmax of 4.5 hours.
Effect of Food
Administration of RELYVRIO in the presence of a high-fat meal resulted in both significantly slower absorption (Cmax reduced by 76%) and lower overall exposure (AUC reduced by 54%) of sodium phenylbutyrate. A high-fat meal did not significantly affect the Cmax for taurursodiol, but AUC was increased by 39% [see Dosage and Administration (2.2)].
Distribution
Human plasma protein binding for sodium phenylbutyrate and taurursodiol is 82% and 98%, respectively.
Elimination
Metabolism
No mass balance studies of sodium phenylbutyrate and taurursodiol have been conducted in humans to confirm the metabolic pathways and elimination routes. Phenylacetate was found to be a major metabolite of phenylbutyrate. Ursodiol and glyco-ursodiol were found as major metabolites of taurursodiol.
Excretion
The majority of administered sodium phenylbutyrate (~80-100%) is excreted in the urine within 24 hours as the conjugated product, phenylacetylglutamine.
Specific Populations
The effect of age, gender, racial, or ethnic groups on the pharmacokinetics of RELYVRIO is unknown.
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of RELYVRIO has not been studied. There were no reports of safety issues with patients with mild renal impairment who were enrolled in Study 1. However, there is no clinical experience for subjects with moderate and severe renal impairment [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of RELYVRIO has not been studied. There were no reports of safety issues with patients with mild hepatic impairment (using National Cancer Institute Classification system) who were enrolled in Study 1. However, there is no clinical experience for subjects with moderate and severe hepatic impairment [see Use in Specific Populations (8.7)].
Drug Interaction Studies [see Drug Interactions (7.1, 7.2)]
No clinical interaction studies between RELYVRIO and other medicinal products have been performed.
In vitro studies showed that the combination of sodium phenylbutyrate and taurursodiol:
- induces CYP1A2, CYP2B6, and CYP3A4 at clinically relevant concentrations
- inhibits CYP2C8 and CYP2B6 at clinically relevant concentrations.
- inhibits OAT1, P-gP, and BCRP at clinically relevant concentrations
- is a substrate of OATP1B3, MATE2-K, OAT3, and BSEP
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Studies to assess the carcinogenic potential of RELYVRIO have not been conducted.
Mutagenesis
The combination of sodium phenylbutyrate and taurursodiol (3:1 ratio of sodium phenylbutyrate and taurursodiol) was negative in in vitro (bacterial reverse mutation and mammalian cell chromosomal aberration) and in vivo (mouse micronucleus) assays.
Impairment of Fertility
Oral administration of the combination of sodium phenylbutyrate and taurursodiol (0, 375, 750, or 1500 mg/kg/day) prior to and throughout mating in male and female rats and continuing to gestation day 7 in females resulted in no adverse effects on fertility. At the highest dose of the combination of sodium phenylbutyrate and taurursodiol tested, doses of sodium phenylbutyrate and taurursodiol were approximately 2 times the maximum recommended doses (6 g sodium phenylbutyrate and 2 g taurursodiol) in humans, based on body surface area (mg/m2).
14 CLINICAL STUDIES
14.1 Clinical Efficacy
The efficacy of RELYVRIO for the treatment of ALS was demonstrated in a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study that evaluated RELYVRIO in adult patients with amyotrophic lateral sclerosis (ALS) (Study 1; NCT03127514). For inclusion in the study, patients had to have a definite diagnosis of sporadic or familial ALS as defined by the revised El Escorial criteria, with symptom onset within the past 18 months, and a slow vital capacity (SVC) greater than 60% of predicted at screening.
A total of 137 patients were randomized 2:1 to receive either RELYVRIO (n=89) or placebo (n=48) for 24 weeks (Intent-to-Treat [ITT] population).
Baseline disease characteristics were generally comparable between the two treatment groups; 95% were Caucasian, the median age was 57.7 years, and 68% were males. Thirty percent of patients in the RELYVRIO treatment group had bulbar disease onset vs. 21% in the placebo group. On average, patients had been diagnosed with ALS six months prior to baseline with a time since onset of first symptom of approximately 13.5 months. At or prior to study entry, 71% of patients were taking riluzole and 34% were taking edaravone. The average (standard deviation) baseline ALS Functional Rating Scale-Revised (ALSFRS-R) total score was 35.7 (5.8) in the RELYVRIO treatment group and 36.7 (5.1) in the placebo group.
Patients were administered the contents of one packet of RELYVRIO or placebo, once daily for the first 3 weeks. After 3 weeks of treatment, the dose was increased to one packet twice daily if tolerated.
The prespecified primary efficacy endpoint was a comparison of the rate of reduction in the ALSFRS-R total scores from baseline to Week 24 in the mITT population. The ALSFRS-R scale consists of 12 questions that evaluate the fine motor, gross motor, bulbar, and respiratory function of patients with ALS (speech, salivation, swallowing, handwriting, cutting food, dressing/hygiene, turning in bed, walking, climbing stairs, dyspnea, orthopnea, and respiratory insufficiency). Each item is scored from 0-4, with higher scores representing greater functional ability.
There was a statistically significant difference in the rate of reduction in the ALSFRS-R total score from baseline to Week 24 in RELYVRIO-treated patients compared to placebo-treated patients (p = 0.034) (see Table 2).
| Treatment | LS Mean (SE) ALSFRS-R Total Score at Week 24 |
Treatment Difference (SE) | p-value |
| RELYVRIO | 29.06 (0.781) | 2.32 points (1.094) [95% CI: 0.18, 4.47] | 0.034 |
| Placebo | 26.73 (0.975) |
In a post hoc, long-term survival analysis, vital status was ascertained in 136 of 137 patients who were enrolled in Study 1. Longer median overall survival was observed in the patients originally randomized to RELYVRIO compared to those originally randomized to placebo. This exploratory analysis should be interpreted cautiously given the limitations of data collected outside of a controlled study, which may be subject to confounding.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
RELYVRIO for oral suspension is supplied in single-dose packets of white to yellow powder containing 3 g sodium phenylbutyrate and 1 g taurursodiol as follows:
- Carton of 7 single-dose packets (NDC 73063-035-04)
- Carton of 56 single-dose packets (Carton NDC 73063-035-03), contained in 4 boxes with 14 single-dose packets per box
16.2 Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from moisture. See Dosage and Administration (2.2) for storage of RELYVRIO reconstituted suspension.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration
Instruct patients or caregivers to empty the contents of one packet in a cup containing 8 ounces of room temperature water and stir vigorously. Advise them that RELYVRIO can be taken orally or administered via feeding tube, and to use or discard within 1 hour of preparation. Instruct to administer RELYVRIO before a snack or meal [see Dosage and Administration (2.2)].
Enterohepatic Circulation, Pancreatic and Intestinal Disorders
Inform patients about the risks and benefits with the use of RELYVRIO if they have underlying medical conditions such as enterohepatic circulation, pancreatic, or intestinal disorders and advise them to notify their healthcare provider if they have new or worsening diarrhea [see Warnings and Precautions (5.1)].
Sodium Intake
Inform patients that 2 packets of RELYVRIO contain 928 mg sodium (46% of WHO daily recommended intake) and patients who are sensitive to sodium (e.g., those with congestive heart failure, severe renal insufficiency, or other conditions associated with sodium retention) should limit their sodium intake [see Warnings and Precautions (5.2)].
Aluminum-based Antacids
Inform patients that aluminum-based antacids may interfere with the absorption of RELYVRIO, and therefore should not be taken during treatment with RELYVRIO [see Drug Interactions (7.1)].
Pregnancy and Breastfeeding
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during RELYVRIO therapy [see Use in Specific Populations (8.1)].
Advise patients to notify their healthcare provider if they intend to breastfeed or are breastfeeding an infant [see Use in Specific Populations (8.2)].
Manufactured for and Distributed by:
Amylyx Pharmaceuticals, Inc., 43 Thorndike Street, Cambridge, MA 02141
©2022 Amylyx Pharmaceuticals, Inc. All Rights Reserved. AMYLYX® and RELYVRIO™ are trademarks of Amylyx Pharmaceuticals, Inc.
|
PATIENT INFORMATION RELYVRIO (Re-LIV-rio) |
|||
What is RELYVRIO?
|
|||
|
Before taking RELYVRIO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements, and any taurursodiol products, such as tauroursodeoxycholic acid (TUDCA). |
|||
How should I take RELYVRIO?
|
|||
|
What are the possible side effects of RELYVRIO? RELYVRIO may cause serious side effects, including:
The most common side effects of RELYVRIO include: |
|||
|
|
|
|
| Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of RELYVRIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
How should I store RELYVRIO?
|
|||
| General information about the safe and effective use of RELYVRIO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use RELYVRIO for a condition for which it was not prescribed. Do not give RELYVRIO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about RELYVRIO that is written for health professionals. |
|||
|
What are the ingredients in RELYVRIO? Active ingredients: sodium phenylbutyrate, taurursodiol Inactive ingredients: acacia, dibasic sodium phosphate, maltodextrin, medium-chain triglycerides, mixed berry flavoring, other flavoring ingredients, silicon dioxide, sodium stearyl fumarate, sorbitol, and sucralose. Marketed and distributed by: Amylyx Pharmaceuticals, Inc., 43 Thorndike St., Cambridge, MA 02141 For more information, go to AMYLYX.com or call 1-877-374-1208. ©2022 Amylyx Pharmaceuticals, Inc. All Rights Reserved. AMYLYX® and RELYVRIO™ are trademarks of Amylyx Pharmaceuticals, Inc. |
|||
| This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 9/2022 | |||