FULL PRESCRIBING INFORMATION
WARNING: INCREASED RISK FOR MORTALITY WHEN USED FOR LONGER DURATION
An increased incidence of mortality was seen in TEMBEXA-treated subjects compared to placebo-treated subjects in a 24-week clinical trial when TEMBEXA was evaluated in another disease [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
1.1 Treatment of Human Smallpox Disease
TEMBEXA® is indicated for the treatment of human smallpox disease caused by variola virus in adult and pediatric patients, including neonates.
1.2 Limitations of Use
TEMBEXA is not indicated for the treatment of diseases other than human smallpox disease [see Warnings and Precautions (5.1, 5.2)].
The effectiveness of TEMBEXA for the treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible, and inducing smallpox disease in humans to study the drug’s efficacy is not ethical [see Clinical Studies (14)].
TEMBEXA efficacy may be reduced in immunocompromised patients based on studies in immune deficient animals.
2 DOSAGE AND ADMINISTRATION
2.1 Testing Before Initiating and During Treatment with TEMBEXA
Perform hepatic laboratory testing in all patients before starting TEMBEXA and while receiving TEMBEXA, as clinically appropriate [see Warnings and Precautions (5.2) and Use in Specific Populations (8.7)].
Perform pregnancy testing before initiation of TEMBEXA in individuals of childbearing potential to inform risk [see Warnings and Precautions (5.5) and Use in Specific Populations (8.3)].
2.2 Important Administration Instructions
Avoid direct contact with broken or crushed tablets or oral suspension. If contact with skin or mucous membranes occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with water [see Warnings and Precautions (5.6)].
TEMBEXA Tablets
TEMBEXA tablets can be taken on an empty stomach or with a low-fat meal (approximately 400 calories, with approximately 25% of calories from fat) [see Clinical Pharmacology (12.3)]. Swallow TEMBEXA tablets whole. Do not crush or divide TEMBEXA tablets.
TEMBEXA Oral Suspension
Take TEMBEXA oral suspension on an empty stomach [see Clinical Pharmacology (12.3)]. Shake oral suspension before use. Use an appropriate oral dosing syringe to correctly measure the total prescribed dose [see Patient Counseling Information (17)]. Discard unused portion after completion of 2 prescribed doses.
For patients who cannot swallow, TEMBEXA oral suspension can be administered by enteral tube (naso-gastric or gastrostomy tubes) as follows:
- •
- Draw up prescribed dose with a calibrated catheter-tip syringe, and utilize this syringe to administer the dose via the enteral tube.
- •
- Refill the catheter-tip syringe with 3 mL of water, shake, and administer the contents via the enteral tube.
- •
- Flush with water before and after enteral administration.
2.3 Recommended Dosage
The recommended dosage of TEMBEXA in pediatric and adult patients is displayed in Table 1 [see Drug Interactions (7.1), Clinical Pharmacology (12.3) and Clinical Studies (14)].
|
Patient’s Weight (kg) |
TEMBEXA Oral Suspension (10 mg/mL) |
TEMBEXA Tablet (100 mg) |
|
Less than 10 kg |
6 mg/kg once weekly for 2 doses (on Days 1 and 8) |
N/A |
|
10 kg to less than 48 kg |
4 mg/kg once weekly for 2 doses (on Days 1 and 8) |
N/A |
|
48 kg and above |
200 mg (20 mL) once weekly for 2 doses (on Days 1 and 8) |
200 mg (two 100 mg tablets) once weekly for 2 doses |
3 DOSAGE FORMS AND STRENGTHS
Tablets
TEMBEXA tablets are blue, modified-oval shape, film-coated tablets debossed with BCV on one side and 100 on the other side. Each tablet contains 100 mg of brincidofovir.
Oral Suspension
TEMBEXA oral suspension is an aqueous based, preserved white to off-white opaque, lemon lime flavored suspension containing 10 mg/mL of brincidofovir.
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk for Mortality When Used for Longer Duration
TEMBEXA is not indicated for use in diseases other than human smallpox. An increase in mortality was observed in a randomized, placebo-controlled Phase 3 trial when TEMBEXA was evaluated in another disease. An increased risk in mortality is possible if TEMBEXA is used for a duration longer than at the recommended dosage on Days 1 and 8 [see Indications and Usage (1.2) and Dosage and Administration (2.3)].
Study 301 (CMX001-301) evaluated TEMBEXA versus placebo for the prevention of cytomegalovirus infection. A total of 303 subjects received TEMBEXA (100 mg twice weekly) and 149 subjects received matching placebo for up to 14 weeks. The primary endpoint was evaluated at Week 24. All-cause mortality at Week 24 was 16% in the TEMBEXA group compared to 10% in the placebo group. Safety and effectiveness of TEMBEXA have not been established for diseases other than human smallpox disease.
5.2 Elevations in Hepatic Transaminases and Bilirubin
Elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin have been observed, including cases of concurrent increases in ALT and bilirubin. During the first 2 weeks of TEMBEXA therapy in 392 subjects, ALT elevations >3x the upper limit of normal were reported in 7% of subjects and bilirubin elevations >2x the upper limit of normal were reported in 2% of subjects; these elevations in hepatic laboratory tests were generally reversible and did not require discontinuation of TEMBEXA [see Adverse Reactions (6.1) and Nonclinical Toxicology (13.2)]. Severe hepatobiliary adverse events including hyperbilirubinemia, acute hepatitis, hepatic steatosis, and venoocclusive liver disease have been reported in less than 1% of subjects.
Perform hepatic laboratory testing in all patients before starting TEMBEXA and while receiving TEMBEXA, as clinically appropriate. Monitor patients who develop abnormal hepatic laboratory tests during TEMBEXA therapy for the development of more severe hepatic injury. Consider discontinuing TEMBEXA if ALT levels remain persistently >10x the upper limit of normal. Do not give the second and final dose of TEMBEXA on Day 8 if ALT elevation is accompanied by clinical signs and symptoms of liver inflammation or increasing direct bilirubin, alkaline phosphatase, or International Normalized Ratio (INR) [see Adverse Reactions (6.1) and Drug Interactions (7.1)].
5.3 Diarrhea and Other Gastrointestinal Adverse Events
During the first 2 weeks of TEMBEXA therapy in 392 subjects, a composite term of diarrhea (all grade, all cause) occurred in 40% of TEMBEXA-treated subjects compared with 25% of subjects in the placebo control group. Treatment with TEMBEXA was discontinued in 5% of subjects for diarrhea (composite term) compared with 1% in the placebo control group. Additional gastrointestinal (GI) adverse events included nausea, vomiting, and abdominal pain; some of these adverse events required discontinuation of TEMBEXA [see Adverse Reactions (6.1) and Drug Interactions (7.1) and Nonclinical Toxicology (13.2)].
Monitor patients for GI adverse events including diarrhea and dehydration, provide supportive care, and if necessary, do not give the second and final dose of TEMBEXA.
5.4 Coadminstration with Related Products
TEMBEXA should not be co-administered with intravenous cidofovir. Brincidofovir, a lipid-linked derivative of cidofovir, is intracellularly converted to cidofovir [see Clinical Pharmacology (12.3)].
5.5 Embryo-fetal Toxicity
Based on findings from animal reproduction studies, TEMBEXA may cause fetal harm when administered to pregnant individuals. TEMBEXA administration to pregnant rats and rabbits resulted in embryotoxicity, decreased embryo-fetal survival and/or structural malformations. These effects occurred in animals at systemic exposures less than the expected human exposure based on the recommended dose of TEMBEXA. Use an alternative therapy to treat smallpox during pregnancy, if feasible. Perform pregnancy testing in individuals of childbearing potential before initiation of TEMBEXA. Advise individuals of childbearing potential to avoid becoming pregnant and to use effective contraception during treatment with TEMBEXA and for at least 2 months after the last dose. Advise individuals of reproductive potential with partners of childbearing potential to use condoms during treatment with TEMBEXA and for at least 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.6 Carcinogenicity
TEMBEXA is considered a potential human carcinogen. Mammary adenocarcinomas and squamous cell carcinomas occurred in rats at systemic exposures less than the expected human exposure based on the recommended dose of TEMBEXA [see Nonclinical Toxicology (13.1)]. Do not crush or divide TEMBEXA tablets. Avoid direct contact with broken or crushed tablets or oral suspension. If contact with skin or mucous membranes occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with water [see How Supplied/Storage and Handling (16)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Elevations in hepatic transaminases and bilirubin [see Warnings and Precautions (5.2)]
- •
- Diarrhea and other GI adverse events [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of TEMBEXA has not been studied in patients with smallpox disease.
The safety of TEMBEXA was evaluated in 392 adult subjects aged 18 to 77 years in Phase 2 and 3 randomized, placebo-controlled clinical trials. Of the subjects who received a 200 mg total weekly dose of TEMBEXA, 54% were male, 85% were White, 7% were Black/African American, 6% were Asian, and 10% were Hispanic or Latino. Twenty-one percent of subjects in the studies were age 65 or older. Of these 392 subjects, 85% received a 200 mg total weekly dose of TEMBEXA for at least 2 weeks.
Common Adverse Reactions
The most common adverse reactions (adverse events assessed as causally related by the investigator) experienced in the first 2 weeks of dosing with TEMBEXA were diarrhea and nausea. Adverse reactions that occurred in at least 2% of subjects in the TEMBEXA treatment group are shown in Table 2.
| Note: Only adverse reactions with onset in the first 2 weeks of treatment are presented. a Composite term includes: bowel movement irregularity, defecation urgency, diarrhea, fecal incontinence, and frequent bowel movements. b Composite term includes: vomiting and retching. c Composite term includes: abdominal discomfort, abdominal distention, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness, and gastrointestinal pain. |
||
|
Adverse Reaction |
TEMBEXA 200 mg
|
Placebo
|
|
Diarrheaa |
8 |
3 |
|
Nausea |
5 |
1 |
|
Vomitingb |
4 |
1 |
|
Abdominal painc |
3 |
2 |
Adverse Reactions Leading to Discontinuation of TEMBEXA
Fifteen subjects (4%) had their treatment with TEMBEXA discontinued due to adverse reactions. One subject had two adverse reactions; the other subjects had one reaction each. These adverse reactions were:
- •
- Diarrhea (n=9)
- •
- Nausea (n=3)
- •
- Vomiting (n=1)
- •
- Enteritis (n=1)
- •
- ALT increased (n=1)
- •
- Dyspepsia (n=1)
These adverse reactions were mild (Grade 1, n=1), moderate (Grade 2, n=7) or severe (Grade 3, n=8) in severity and resolved upon discontinuation of TEMBEXA.
Less Common Adverse Reactions
Clinically significant adverse reactions that were reported in <2% of subjects (and also occurred in 2 or more subjects) exposed to TEMBEXA and at rates higher than in subjects who received placebo are listed below:
- •
- General and administration site: peripheral edema
- •
- Metabolism and nutrition: decreased appetite
- •
- Musculoskeletal and connective tissue: muscular weakness
- •
- Nervous system: dysgeusia
- •
- Skin and subcutaneous tissue: rash (includes rash, maculo-papular rash, pruritic rash)
Selected treatment-emergent laboratory values occurring during the first 2 weeks of treatment with TEMBEXA are presented in Table 3.
| ULN = upper limit of normal a Frequencies are based on treatment-emergent laboratory abnormalities. Graded per Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 toxicity grading criteria. b ALT >10x ULN occurred in one subject in the TEMBEXA group and no subjects in the placebo group. c No subjects reported AST >10x ULN. |
||||||
|
Laboratory Parameter Abnormalitya |
TEMBEXA 200 mg
|
Placebo
|
||||
|
Alanine aminotransferase |
n |
382 |
203 |
|||
|
Grade 2 (>3 to 5x ULN), (%) |
3 |
2 |
||||
|
Grade 3 (>5 to 20x ULN), (%) |
2 |
1 |
||||
|
Grade 4 (>20x ULN), (%) |
0 |
0 |
||||
|
Aspartate aminotransferase |
n |
380 |
201 |
|||
|
Grade 2 (>3 to 5x ULN), (%) |
2 |
1 |
||||
|
Grade 3 (>5 to 20x ULN), (%) |
1 |
0 |
||||
|
Grade 4 (>20x ULN), (%) |
0 |
0 |
||||
|
Total bilirubin |
n |
382 |
203 |
|||
|
Grade 2 (>1.5 to 3x ULN), (%) |
3 |
2 |
||||
|
Grade 3 (>3 to 10x ULN), (%) |
1 |
<1 |
||||
|
Grade 4 (>10x ULN), (%) |
0 |
0 |
||||
|
Serum creatinine |
n |
383 |
205 |
|||
|
Grade 2 (>1.5 to 3x ULN), (%) |
4 |
4 |
||||
|
Grade 3 (>3 to 10x ULN), (%) |
<1 |
0 |
||||
|
Grade 4 (>10x ULN), (%) |
0 |
0 |
||||
Adverse Reactions in Pediatric Subjects
In 23 pediatric subjects aged 7 months to 17 years who received TEMBEXA in a randomized, placebo-controlled clinical trial, the adverse reactions and laboratory abnormalities observed with TEMBEXA were similar to adults [see Use in Specific Populations (8.4)].
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on TEMBEXA
Inhibitors for Organic Anion Transporting Polypeptide (OATP) 1B1 and 1B3
Concomitant use of TEMBEXA with OATP1B1 and 1B3 inhibitors (clarithromycin, cyclosporine, erythromycin, gemfibrozil, human immunodeficiency virus [HIV] and hepatitis C virus [HCV] protease inhibitors, rifampin [single dose]) increase brincidofovir AUC and Cmax which may increase TEMBEXA-associated adverse reactions [see Clinical Pharmacology (12.3)].
Where possible, consider alternative medications that are not OATP1B1 or 1B3 inhibitors. If concomitant use with TEMBEXA is necessary, increase monitoring for adverse reactions associated with TEMBEXA (elevations in transaminases and bilirubin, diarrhea, or other GI adverse events) [see Warnings and Precautions (5.2, 5.3)] and postpone the dosing of OATP1B1 or 1B3 inhibitors for at least 3 hours after TEMBEXA administration.
7.2 Vaccine Interactions
No vaccine-drug interaction studies have been performed in human subjects. Animal studies have indicated that coadministration of TEMBEXA at the same time as live smallpox vaccine (vaccinia virus) may reduce the immune response to the vaccine. It is also possible that TEMBEXA may reduce the immune response to replication-defective smallpox vaccine (modified vaccinia virus Ankara). The clinical impacts of these potential interactions on vaccine efficacy are unknown.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal reproduction studies, TEMBEXA may cause fetal harm when administered to pregnant individuals. Use an alternative therapy to treat smallpox during pregnancy, if feasible. There are no available data on the use of brincidofovir in pregnant individuals to evaluate for a drug-associated risk of major birth defects, miscarriage, and other adverse maternal and fetal outcomes. In animal reproduction studies, oral administration of brincidofovir to pregnant rats and rabbits during the period of organogenesis resulted in embryotoxicity and structural malformations. These effects occurred in animals at systemic exposures less than the expected human exposure based on the recommended dose of TEMBEXA (see Data).
The estimated background risk of major birth defects for the indicated population is unknown, and the estimated background risk of miscarriage for the indicated population is higher than the general population. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals were administered oral doses of brincidofovir up to 4.5 mg/kg/day from gestation days 7 to 20. Maternal toxicity in rats, characterized by decreases in food consumption and decreased body weight gain, was observed at doses of 1.5 and 4.5 mg/kg/day. These effects correlated with decreased fetal weights in rats given 4.5 mg/kg/day. Brincidofovir administration in rats was not associated with effects on intrauterine growth or survival at any dose, and there were no external malformations or developmental variations.
In rabbits, 4.5 mg/kg/day brincidofovir was associated with decreased maternal body weight and food consumption, decreased fetal body weight, increased late resorptions and morphological changes which included external, visceral and skeletal malformations and variations.
In the pre-/post-natal development study, administration of brincidofovir at doses of 0, 0.25, 1, and 4 mg/kg/day and 15 mg/kg twice weekly to pregnant rats from gestation day 7 to lactation day 20 resulted in pup toxicity at maternally toxic doses (4 mg/kg/day and 15 mg/kg twice weekly). Pup body weight and viability were decreased, and reproductive function of the pups was impaired as evidenced by a delay in sexual maturation, decreased testes and epididymal size, reduced mating and an increase in the number of days to mating as well as preimplantation loss.
All effects were observed at systemic exposures less than the expected human exposure based on the recommended dose of TEMBEXA.
8.2 Lactation
Risk Summary
Because of the potential for variola virus transmission through direct contact with the breastfed infant, breastfeeding is not recommended in patients with smallpox. There are no data on the presence of brincidofovir in human milk, the effects of the drug on the breastfed infant, or on milk production. Brincidofovir is present in animal milk (see Data).
8.3 Females and Males of Reproductive Potential
Based on animal data, TEMBEXA may cause fetal harm [see Use in Specific Populations (8.1)].
Pregnancy Testing
Perform pregnancy testing in individuals of childbearing potential before initiation of TEMBEXA [see Dosage and Administration (2.1) and Warnings and Precautions (5.5)].
Contraception
Females
Advise individuals of childbearing potential to use effective contraception during treatment and for at least 2 months after the last dose of TEMBEXA [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)].
Males
Advise sexually active individuals with partners of childbearing potential to use condoms during treatment and for at least 4 months after last dose of TEMBEXA.
Infertility
Males
Based on testicular toxicity in animal studies, TEMBEXA may irreversibly impair fertility in individuals of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
As in adults, the effectiveness of TEMBEXA in smallpox infected pediatric patients, including neonates, is based solely on efficacy studies in animal models of orthopoxvirus disease. The recommended pediatric dosing regimen is expected to produce brincidofovir exposures that are comparable to those in adults based on a population pharmacokinetic modeling and simulation approach. The dosage for pediatric patients is based on weight [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
There have been 23 pediatric subjects aged 7 months to 17 years who received TEMBEXA in a randomized, placebo-controlled clinical trial. The safety in adult and pediatric subjects treated with TEMBEXA were similar [see Adverse Reactions (6.1)]. An additional 166 pediatric subjects aged 3 months to 18 years of age received TEMBEXA from uncontrolled studies and expanded access. The dosage of TEMBEXA in pediatric patients <3 months of age was based on modeling and simulations [see Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Of the 392 subjects in the controlled clinical studies, 21% were ≥65 years of age and 1% were ≥75 years of age. The nature and severity of adverse events was comparable between subjects older and younger than 65 years. No alteration of dosing is recommended for patients ≥65 years of age [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment of TEMBEXA is required for patients with mild, moderate, or severe renal impairment or patients with end stage renal disease (ESRD) receiving dialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Perform hepatic laboratory testing in all patients before starting TEMBEXA and while receiving TEMBEXA, as clinically appropriate. No dosage adjustment is required for patients with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, or C) [see Warnings and Precautions (5.2), Adverse Reactions (6.1), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is no clinical experience with overdosage of TEMBEXA. In the event of an overdose, monitor patients for adverse effects and provide appropriate supportive care.
11 DESCRIPTION
TEMBEXA (brincidofovir) tablets, 100 mg, for oral use are immediate release film-coated tablets containing the following inactive ingredients: Colloidal Silicon Dioxide, Crospovidone, FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, Magnesium Stearate, Mannitol, Microcrystalline Cellulose, Polyethylene Glycol, Polyvinyl Alcohol, Purified Water, Silicified Microcrystalline Cellulose, Talc and Titanium Dioxide.
TEMBEXA (brincidofovir) oral suspension, 10 mg/mL, is an aqueous based, preserved, orally dosed suspension. The inactive ingredients are: Citric Acid Anhydrous, Lemon Lime Flavor, Microcrystalline Cellulose and Carboxymethyl Cellulose Sodium, Purified Water, Simethicone 30% Emulsion, Sodium Benzoate, Sucralose, Trisodium Citrate Anhydrous, and Xanthan Gum.
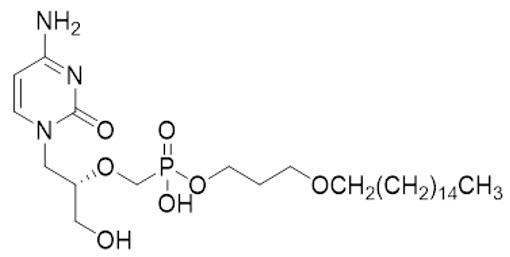
Brincidofovir is an orthopoxvirus nucleotide analog DNA polymerase inhibitor and a lipid conjugate of the nucleotide analog cidofovir and is indicated for the treatment of human smallpox disease. The full chemical name is: Phosphonic acid, P-[[(1S)-2-(4-amino-2-oxo-1(2H)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]-, mono[3-(hexadecyloxy)propyl] ester.
The molecular formula of brincidofovir is C27H52N3O7P and the relative molecular mass is 561.70.
The structure is shown below.
Brincidofovir is a white to off-white crystalline powder as a free acid and practically insoluble in water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Brincidofovir is an antiviral drug against variola (smallpox) virus [see Microbiology (12.4)].
12.2 Pharmacodynamics
Brincidofovir and its active metabolite cidofovir diphosphate exposure-response relationships and time course of pharmacodynamic responses are unknown.
Cardiac Electrophysiology
TEMBEXA does not prolong the QT interval at the anticipated therapeutic exposure.
12.3 Pharmacokinetics
Brincidofovir is a prodrug that is converted intracellularly to cidofovir, which is subsequently phosphorylated to cidofovir diphosphate, the active antiviral moiety, following oral administration. Brincidofovir plasma exposures do not accumulate after repeat doses. The metabolite cidofovir diphosphate reaches maximum concentration at 47 hours (23 to 311 hours) following administration of the recommended dose, with a mean (CV%) half-life of 113 hours (34.2%). The pharmacokinetic properties of brincidofovir following administration of TEMBEXA are provided in Table 4. The pharmacokinetic parameters of brincidofovir and cidofovir diphosphate following administration of TEMBEXA at the recommended dose are provided in Table 5 .
| a Healthy adults. b Administered under fasted conditions. c Low-fat meal: ~400 calories, with ~25% calories from fat. No clinically meaningful change in intracellular concentrations of cidofovir diphosphate were seen when TEMBEXA Tablet was administered with a low-fat meal. The effect of food on TEMBEXA oral suspension has not been studied. d Following administration of radiolabeled brincidofovir. |
||
|
Absorption | ||
|
Bioavailability |
Oral suspension |
16.8% |
|
Tablet |
13.4% |
|
|
Tmaxb |
3 hours (2 to 8 hours) |
|
|
Effect of food on TEMBEXA Tablet (relative to fasting)c |
|
|
|
Distribution | ||
|
% Bound to human plasma proteins |
>99.9% |
|
|
Blood-to-plasma ratio (drug or drug-related materials)d |
0.48 to 0.61 |
|
|
Apparent Volume of distribution, L |
1230 |
|
|
Elimination | ||
|
Apparent Clearance, L/hr |
44.1 |
|
|
Mean terminal half-life (t1/2), hr |
19.3 |
|
|
Metabolism | ||
|
Metabolic pathways |
hydrolysis, CYP4F2 |
|
|
Metabolites |
cidofovir and cidofovir diphosphate (active) |
|
|
Excretion | ||
|
% of dose excreted in urined |
51%, as metabolites |
|
|
% of dose excreted in fecesd |
40%, as metabolites |
|
| AUC = area under the time concentration curve; Cmax = maximum concentration; CV = coefficient of variation. a Healthy adults |
|||||
|
PK Parameter |
Geometric Mean (%CV) |
||||
|
Brincidofovir |
Cidofovir diphosphate |
||||
|
Cmax |
480 ng/mL (70%) |
9.7 pg/106 cells (75%) |
|||
|
AUCtau |
3400 ng·hr/mL (58%) |
1200 pg·hr/106 cells (75%) |
|||
Metabolism
Brincidofovir is metabolized by hydrolysis of the phosphoester bond to form cidofovir. Cidofovir is subsequently phosphorylated to form cidofovir diphosphate. Brincidofovir is also carboxylated at the terminal carbon by Cytochrome P450 (CYP) 4F2, followed by subsequent CYP-mediated oxidations and multiple cycles of fatty acid beta-oxidation. The major inactive metabolites formed via these pathways are CMX103 (3-hydroxypropyl ester of cidofovir) and CMX064 (4-(3-propoxy)butanoic acid ester of cidofovir).
Genetic and chemical inhibition of acid sphingomyelinase enzyme activity in multiple human cell lines resulted in substantially lower concentrations of cidofovir and cidofovir diphosphate (the active drug), compared to controls with functional acid sphingomyelinase enzyme activity. Findings show acid sphingomyelinase plays a major role in the hydrolysis of brincidofovir to cidofovir in these cell lines. Based on in vitro data, acid sphingomyelinase deficiency may reduce the ability to convert brincidofovir to cidofovir and cidofovir diphosphate; however, the clinical relevance of this finding is unknown.
Comparison of Animal and Human Pharmacokinetic Data to Support Effective Human Dose Selection
Because the effectiveness of TEMBEXA cannot be tested in humans, a comparison of brincidofovir and cidofovir diphosphate exposures achieved in human subjects to those observed in animal models of orthopoxvirus infection (rabbits infected with rabbitpox virus, and mice infected with ectromelia virus) in efficacy studies was necessary to support the dose and regimen of 200 mg once a week for 2 doses for treatment of smallpox disease in humans. Humans achieve greater systemic exposures (AUC and Cmax) of brincidofovir and greater than or equal to intracellular concentrations of cidofovir diphosphate following a 200 mg once a week dose when compared with therapeutic exposure in the animal models [see Clinical Studies (14)].
Specific Populations
No clinically meaningful differences in the pharmacokinetics of brincidofovir were observed based on age, sex, race, reduced activity in the CYP4F2 enzyme, renal impairment including ESRD with or without dialysis (based on estimated glomerular filtration rate [GFR]), or hepatic impairment (Child-Pugh Class B, C).
Patients Requiring Hemodialysis
The AUC and Cmax of brincidofovir and its metabolite cidofovir were comparable between subjects requiring hemodialysis whether on- or off-dialysis.
Pediatric Patients
The pharmacokinetics of TEMBEXA suspension has been evaluated in pediatric individuals. Pharmacokinetic simulation was used to derive dosing regimens that are predicted to provide pediatric patients, including neonates, with exposures comparable to the observed exposure in adults receiving TEMBEXA tablets.
Drug Interaction Studies
Clinical Studies
OATP1B1 and 1B3 Inhibitors: A single 600 mg oral cyclosporine (OATP1B1 and 1B3 inhibitor) dose increased the mean brincidofovir AUC0-inf and Cmax by 374% and 269%, respectively, when administered concomitantly with TEMBEXA.
CYP Substrates: No clinically significant differences in the pharmacokinetics of midazolam (sensitive CYP3A substrate) were observed when administered concomitantly with TEMBEXA.
P-gp Substrates: No clinically significant differences in the pharmacokinetics of dabigatran etexilate (P-gp substrate) were observed when administered concomitantly with TEMBEXA.
In vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically
CYP Enzymes: Brincidofovir is a direct and reversible inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP4F2. Brincidofovir is not an inducer of CYP1A2, CYP2B6 or CYP3A.
Transporter Systems: Brincidofovir is an inhibitor of Breast Cancer Resistance Protein (BCRP), multidrug resistance-associated protein 2 (MRP2), bile salt export pump (BSEP), OATP1B1, Organic Anion Transporter 1 (OAT1), and OAT3. Brincidofovir is not an inhibitor of OATP1B3, Organic Cation Transporter 2 (OCT2), multidrug and toxin extrusion protein 1 (MATE1), or MATE2-K in vitro.
12.4 Microbiology
Mechanism of Action
Brincidofovir is a lipid conjugate of cidofovir, an acyclic nucleotide analog of deoxycytidine monophosphate. The lipid conjugate is designed to mimic a natural lipid, lysophosphatidylcholine, and thereby use endogenous lipid uptake pathways. Once inside cells, the lipid ester linkage of brincidofovir is cleaved to liberate cidofovir, which is then phosphorylated to produce the active antiviral, cidofovir diphosphate. Based on biochemical and mechanistic studies using recombinant vaccinia virus E9L DNA polymerase, cidofovir diphosphate selectively inhibits orthopoxvirus DNA polymerase-mediated viral DNA synthesis. Incorporation of cidofovir into the growing viral DNA chain results in reductions in the rate of viral DNA synthesis.
Activity in Cell Culture
The median 50% effective concentration (EC50) of brincidofovir against variola virus was 0.11 μM (range 0.05 to 0.21 μM) across 5 variola virus strains chosen to represent 5 distinct variola virus DNA polymerase genotypes. The median EC50 values of brincidofovir against rabbitpox, ectromelia, vaccinia, and monkeypox viruses were 1.10 μM (n=4, 0.5-1.89 μM), 0.33 μM (n=5, 0.12-0.51 μM), 0.17 μM (n=22, 0.004-1.2 μM), and 0.074 μM (n=2, 0.023-0.12 μM), respectively.
Non-antagonistic antiviral activity of brincidofovir and tecovirimat has been demonstrated in cell culture and animal models.
Resistance
There are no known instances of naturally occurring brincidofovir resistant orthopoxviruses, although brincidofovir resistance may develop under drug selection. Cell culture studies have shown that certain amino acid substitutions in the target viral DNA polymerase protein can confer reductions in brincidofovir antiviral activity. The possibility of resistance to brincidofovir should be considered in patients who either fail to respond to therapy or who develop recrudescence of disease after an initial period of responsiveness.
Cross-resistance
Cross-resistance between brincidofovir and tecovirimat is not expected based on their distinct mechanisms of action. Where tested, orthopoxvirus isolates resistant to tecovirimat have not been resistant to brincidofovir and/or cidofovir and vice versa.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Palpable masses occurred in rats with high frequency after as few as 26 oral doses of brincidofovir at systemic exposures less than the expected human exposure based on the recommended dose of TEMBEXA. The masses diagnosed as mammary adenocarcinomas, carcinoma in squamous cell, Zymbal’s gland, uterus and small intestine and hemangiosarcomas in mesenteric and mediastinal lymph node, liver and abdominal cavity were observed in rats following long term (13-weeks and 26-weeks) dosing studies. No tumors occurred in rats after 9 twice-weekly intravenous doses, although rats were only followed for 14 days after the last administration. Based on these data and the unknown translation of nonclinical findings to clinical risk, TEMBEXA is considered a potential human carcinogen.
Brincidofovir was negative in a bacterial mutagenicity (Ames) assay and an in vivo micronucleus assay in mice. Brincidofovir was positive for increased structural chromosomal aberrations in the absence of metabolic activation in an in vitro assay.
Impairment of Fertility
In chronic dosing studies with orally administered brincidofovir, testicular effects were seen in both rats and monkeys. Monkeys administered twice weekly doses of brincidofovir via oral gavage for 9 months exhibited atrophy of the seminiferous tubules and hypospermia in the epididymides. Based on sperm analysis and histopathology, these findings demonstrated a trend towards recovery after a 6 month, post-dosing period. Rats administered brincidofovir via oral gavage twice weekly for 13 weeks exhibited decreased testes weights, depletion of spermatogenesis and hypospermia. Unlike in the monkey, recovery was not demonstrated in the rats following a 12-week post-dosing period.
In a rat fertility and early embryonic development study, administration of brincidofovir once daily beginning 15 days before cohabitation, during cohabitation and continuing through gestation day 7 resulted in decreased embryonal viability at 0.25 mg/kg/day, a dose that did not cause maternal toxicity. Male rats dosed twice weekly by oral gavage for 10 to 19 weeks had reduced sperm motility and a decrease in total sperm count. These effects resulted in reduced fertility during the first cohabitation period, and infertility during the second cohabitation period.
Brincidofovir exposures in both monkeys and rats were less than exposures seen in humans administered 200 mg brincidofovir. Studies conducted using intravenous brincidofovir to achieve clinically relevant exposures demonstrated diminished but ongoing spermatogenesis in the tubules of rats 15 weeks after administration of 3 doses of brincidofovir administered once weekly. The testicular pathology appears to be an effect on the mitotic spermatogonia.
13.2 Animal Toxicology and/or Pharmacology
Gastrointestinal Toxicity: GI toxicity is the dose-limiting toxicity of orally-administered brincidofovir. Signs of GI toxicity include decreased body weight and food consumption, fecal changes (absent, nonformed, or liquid feces), and dehydration. Dose-limiting GI events, diagnosed as gastropathy and enteropathy or enteritis, were observed following daily oral administration of brincidofovir in mice and monkeys. A single dose study to characterize the pathological dynamics of GI toxicity and potential reversibility revealed a dose responsive enteritis in rats, which reversed in surviving animals by 14 days post-dose. Subsequent animal studies used twice weekly oral administration to reflect anticipated clinical use and no dose-limiting GI toxicity was observed.
Aminotransferase Elevations: Increases in ALT (2- to 5-fold) were observed in both rodent and nonrodent species in nonclinical toxicology studies of orally administered brincidofovir. The changes seen with oral dosing appeared with highest frequency in monkeys, followed by mice and then rats. ALT elevations did not correlate with dose concentration and reversed after cessation of dosing. There were no gross or microscopic hepatic changes that correlated with the ALT increases.
14 CLINICAL STUDIES
Overview
The effectiveness of TEMBEXA for treatment of smallpox disease has not been determined in humans because adequate and well-controlled field trials have not been feasible and inducing smallpox disease in humans to study the efficacy of the drug is not ethical. Therefore, the effectiveness of TEMBEXA for treatment of smallpox disease was established based on results of adequate and well-controlled animal efficacy studies of rabbits and mice infected with species specific non-variola orthopoxviruses. Survival rates observed in the animal studies may not be predictive of survival rates in clinical practice.
Study Design
Efficacy studies were conducted in the rabbitpox model (New Zealand White rabbits infected with rabbitpox virus) and the mousepox model (BALB/c mice infected with ectromelia virus).
The primary efficacy endpoint for these studies was survival. Survival was monitored for 4 to 5 times the mean time to death for untreated animals in each model.
In the rabbitpox study, rabbits were lethally challenged intradermally with 600 plaque-forming units of rabbitpox virus; brincidofovir was administered orally with a regimen of 20/5/5 mg/kg (administered every 48 hours for 3 doses) with brincidofovir treatment initiated on 3, 4, 5, or 6 days post-challenge. The timing of brincidofovir dosing was intended to assess efficacy when treatment is initiated after animals have developed clinical signs of disease, specifically fever in rabbits. Clinical signs of disease were evident in some animals at Day 3 post-challenge but were evident in all animals by Day 4 post-challenge.
In the mousepox study, mice were lethally challenged intranasally with 200 plaque-forming units of ectromelia virus; brincidofovir was administered orally with a regimen of 20/5/5 mg/kg or 10/5/5 mg/kg (administered every 48 hours for 3 doses) with brincidofovir treatment initiated on 4, 5, 6, or 7 days post-challenge. All animals had detectable viremia by 4 days post-challenge. In the mousepox model, a clinically evident sign of disease could not be identified to use as a trigger to initiate treatment.
Study Results
Treatment with brincidofovir resulted in statistically significant improvement in survival relative to placebo, except when the 10/5/5 mg/kg regimen was initiated at Day 6 post-challenge in the mousepox study (Table 6).
| a Survival percentage with brincidofovir-treated animals minus survival percentage in placebo-treated animals. Exact confidence intervals are presented. b P-value is from 1-sided Boschloo test compared with placebo. c 20/5/5 mg/kg (fully effective dose in the rabbitpox model) d 10/5/5 mg/kg (fully effective dose in the mousepox model) e P-value is not significant at the one-sided alpha of 0.0125. |
|||||
|
Dose Regimen (mg/kg) |
Treatment Initiation Day |
Survival % (# survived/n) |
Survival Rate Difference
|
p-valueb |
|
|
Placebo |
Brincidofovir |
||||
|
Rabbitpoxc | |||||
|
Day 4 |
90% (26/29) |
61% (36%, 79%) |
<0.0001 |
||
|
Study 1 |
Day 5 |
29% (8/28) |
69% (20/29) |
40% (12%, 63%) |
0.0014 |
|
Day 6 |
69% (20/29) |
40% (12%, 63%) |
0.0014 |
||
|
Mousepoxd | |||||
|
Day 4 |
78% (25/32) |
66% (44%, 82%) |
<0.0001 |
||
|
Study 2 |
Day 5 |
13% (4/32) |
66% (21/32) |
53% (29%, 72%) |
<0.0001 |
|
Day 6 |
34% (11/32) |
22% (1%, 43%) |
0.0233e |
||
16 HOW SUPPLIED/STORAGE AND HANDLING
TEMBEXA Tablets:
Tablets are blue, modified-oval shape, film-coated tablets debossed with BCV on one side and 100 on the other side and packaged into blister cards. Each blister cavity contains one film-coated tablet containing 100 mg of brincidofovir. The blister card is placed in a child-resistant wallet. Each wallet (NDC 79622-010-04) contains one (1) blister card with a total of 4 film-coated tablets.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Do not divide, break, or crush the tablets. Avoid direct contact with broken or crushed tablets. If contact with skin or mucous membranes occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with water [see Warnings and Precautions (5.6)].
TEMBEXA Oral Suspension:
Aqueous based, preserved white to off-white opaque, lemon lime flavored suspension containing 10 mg/mL of brincidofovir (NDC 79622-012-65) packaged into a high density polyethylene bottle with a low density polyethylene press-in bottle adaptor (PIBA) inserted into the bottle. The bottle is capped by a child-resistant closure. Each bottle is filled to deliver 65 mL of brincidofovir.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not freeze.
Avoid direct contact with oral suspension. If contact with skin or mucous membranes occurs, wash thoroughly with soap and water, and rinse eyes thoroughly with water [see Warnings and Precautions (5.6)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Efficacy Based on Animal Models Alone
Inform patients that the efficacy of TEMBEXA is based solely on efficacy studies demonstrating a survival benefit in animals and that the effectiveness of TEMBEXA has not been tested in humans with smallpox disease [see Clinical Studies (14)].
Elevations of Hepatic Transaminases and Bilirubin
Inform patients of the need for liver monitoring before treatment with TEMBEXA and during treatment if signs or symptoms of liver injury occur. Advise patients to report symptoms that may indicate liver injury, including right upper abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.2)].
Diarrhea and Other Gastrointestinal Adverse Events
Inform patients of the risk of diarrhea and other GI adverse events (nausea, vomiting, and abdominal pain) while taking TEMBEXA. Advise patients to inform their healthcare provider if they develop severe diarrhea or other severe GI symptoms [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
Important Drug Interactions
Inform patients that TEMBEXA may interact with some drugs. If concomitant use of OATP1B1 and 1B3 inhibitors with TEMBEXA is necessary, advise patients to postpone the dosing of these medicines for at least 3 hours after TEMBEXA administration [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Embryo-fetal Toxicity
Advise pregnant individuals and individuals of childbearing potential of the risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy. Advise individuals of childbearing potential to use effective contraception during treatment with TEMBEXA and for at least 2 months after the last dose. Due to animal findings of testicular toxicity, advise individuals of reproductive potential with partners of childbearing potential to use condoms during treatment with TEMBEXA and for at least 4 months after the last dose [see Warnings and Precautions (5.5, 5.7) and Use in Specific Populations (8.3)].
Infertility
Advise individuals of reproductive potential that treatment with TEMBEXA may deplete sperm, resulting in infertility [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
Lactation
Instruct individuals with smallpox not to breastfeed their infant because of the risk of passing variola virus to the breastfed infant [see Use in Specific Populations (8.2)].
Important Administration Instructions for Oral Suspension
Instruct patients or caregivers to use an oral dosing syringe to correctly measure the prescribed amount of medication. Oral dosing syringes may be obtained from the pharmacy. Refer to instructions above for administration of TEMBEXA oral suspension through enteral tubes [see Dosage and Administration (2.2)].
Advise patients taking the oral suspension to discard unused portion after completion of the 2 prescribed doses.
Handling
Advise patients not to divide, break, or crush tablets. Advise patients to avoid direct contact with broken or crushed tablets and oral suspension. If contact with skin or mucous membranes occurs, inform patients to wash thoroughly with soap and water, and rinse eyes thoroughly with water [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)].
Manufactured for Chimerix, Inc. by:
TEMBEXA Tablets:
Penn Pharmaceutical Services, Ltd.
Tredegar, Gwent, NP22 3AA, UK
TEMBEXA Oral Suspension:
Cambrex Whippany, Inc.
Whippany, NJ 07981
Patient Package Insert
|
PATIENT INFORMATION |
|
|
TEMBEXA (tem-BEKS-uh)
|
TEMBEXA (tem-BEKS-uh)
|
|
What is TEMBEXA? TEMBEXA is a prescription medicine used to treat smallpox disease caused by a type of virus called variola virus in adults, children, and infants.
|
|
|
Before taking TEMBEXA, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with TEMBEXA causing side effects. Keep a list of your medicines to show your healthcare provider and pharmacist.
|
|
|
How should I take TEMBEXA?
|
|
|
What are the possible side effects of TEMBEXA? TEMBEXA may cause serious side effects, including:
The most common side effects of TEMBEXA include:
TEMBEXA may cause low sperm counts and affect the ability to conceive children. Talk to your healthcare provider if you have concerns about fertility. These are not all the possible side effects of TEMBEXA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store TEMBEXA?
Keep TEMBEXA and all other medicines out of the reach of children. |
|
|
General information about the safe and effective use of TEMBEXA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TEMBEXA for a condition for which it was not prescribed. Do not give TEMBEXA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TEMBEXA that is written for health professionals. |
|
|
What are the ingredients in TEMBEXA? Active ingredient: brincidofovir Inactive ingredients:
|
|
|
Manufactured for Chimerix, Inc. by: TEMBEXA Tablets: TEMBEXA Oral Suspension: For more information, go to www.Chimerix.com or call 1-888-998-2679. |
|
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 06/2021
PRINCIPAL DISPLAY PANEL - Oral Suspension Carton Label
For oral use
Rx Only
TEMBEXA®
brincidofovir
Oral Suspension, 10 mg/mL
Contents: 65 mL
See Full
Prescribing Information
For TEMBEXA Inside.
NDC 79622-012-65
DOSAGE AND
ADMINISTRATION
- •
- Avoid direct contact with
suspension. If contact with
skin or mucous membranes
occurs, wash thoroughly with
soap and water, and rinse
eyes thoroughly with water. - •
- Recommended Dosage:
See prescribing information - •
- Shake the suspension well
before each use. - •
- Use an appropriate oral
dosing syringe to correctly
measure the total prescribed
dose. Only take the volume
prescribed. Discard unused
portion after completion of
two prescribed doses.
Store at 20°C to 25°C
(68°F to 77°F); excursions
permitted from 15°C to 30°C
(59°F to 86°F) [see USP
Controlled Room Temperature].
Protect from freezing.
Date of first opening / / .
Discard unused portion 30 days
after first opening.
Do not use if seal under cap is
broken or missing.
Keep out of reach of children.
Active ingredient:
Brincidofovir
Inactive ingredients:
Citric Acid Anhydrous, Lemon
Lime Flavor, Microcrystalline
Cellulose and Carboxymethyl
Cellulose Sodium, Purified
Water, Simethicone 30%
Emulsion, Sodium Benzoate,
Sucralose, Trisodium Citrate
Anhydrous, Xanthan Gum
TEMBEXA®
brincidofovir
Manufactured for
Chimerix, Inc. by
Cambrex Whippany, Inc.,
Whippany, NJ 07981
© 2020 Chimerix, Inc.
All rights reserved.
CHIMERIX®
2505 Meridian Parkway, Suite 100
Durham, NC 27713
For more information,
visit www.chimerix.com
or call 1-888-998-2679
GTIN 00000000000000
LOT ABCD1234
EXP DEC 20XX
S/N 100000000001