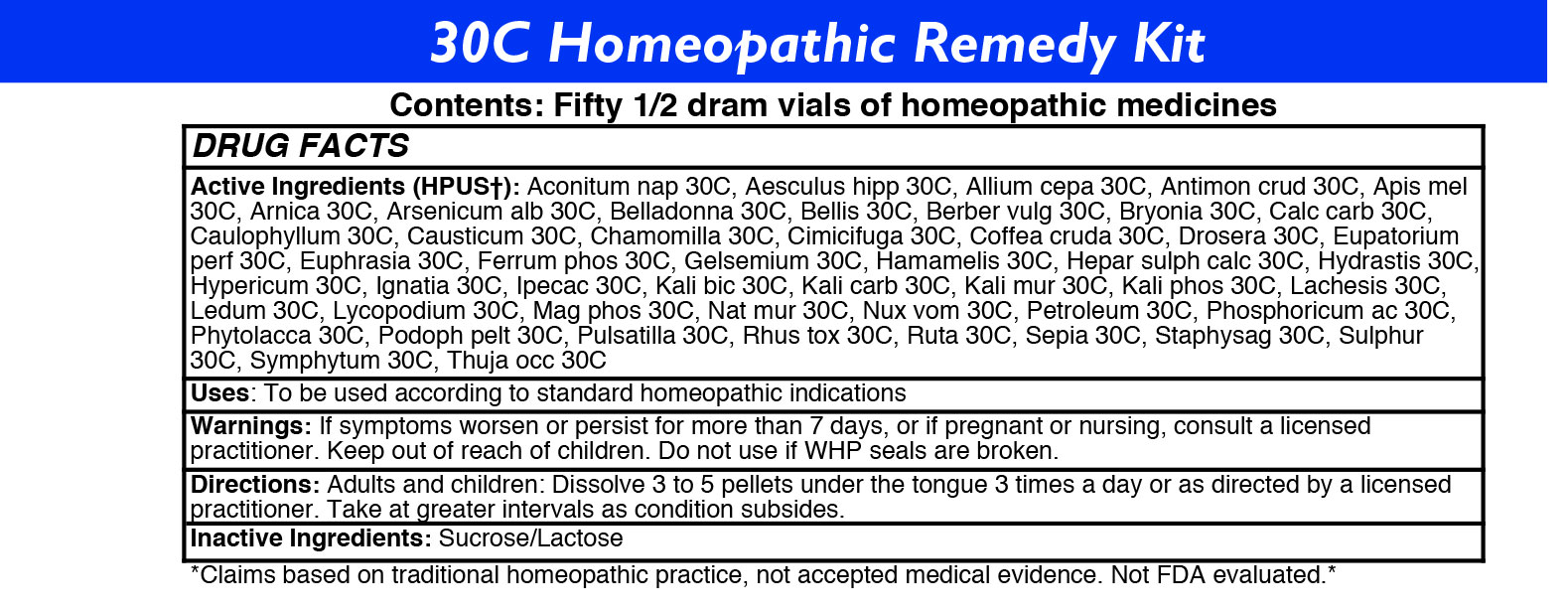
ACTIVE INGREDIENTS
Acon nap
Aesculus hipp
Allium cepa
Antimon crud
Apis mel
Arnica
Arsenicum alb
Belladonna
Bellis
Berber vulg
Bryonia
Calc carb
Caulophyllum
Causticum
Chamomilla
Cimicifuga
Coffea cruda
Drosera
Eupatorium perf
Euphrasia
Ferrum phos
Gelsemium
Hamamelis
Hepar sulph calc
Hydrastis
Hypericum
Ignatia
Ipecac
Kali bic
Kali carb
Kali mur
Kali phos
Lachesis
Ledum
Lycopodium
Mag phos
Nat mur
Nux vom
Petroleum
Phosphoricum ac
Phytolacca
Podoph pelt
Pulsatilla
Rhus tox
Ruta
Sepia
Staphysag
Sulphur
Symphytum
Thuja occ
USES
To relieve symptoms of fear, hemorrhoids, head cold, warts, stings, bruises, vomiting, fever, bruised soreness, itching, worse motion, oberwork, cramps, hoarseness, irritability, minor back pain, sleeplessness, cough, flu-like symptoms, eye irritation, low fever, lethargy, varicose veins, croupiness, sinuses, shooting pain, sadness, nausea, sinuses, sour belching, congested ears, irritability, sore throat, black eyes, digestion, cramps, sneezing, vomiting, nausea, headache, sore throat, diarrhea, weeping, better motion, bruised feeling, indifference, anger, skin problems, prickling pain, and warts.
INDICATIONS
Acon nap fear
Aesculus hipp hemorrhoids
Allium cepa head cold
Antimon crud warts
Apis mel stings
Arnica bruises
Arsenicum alb vomiting
Belladonna fever
Bellis bruised soreness
Berber vulg itching
Bryonia worse motion
Calc carb overwork
Caulophyllum cramps
Causticum hoarseness
Chamomilla irritability
Cimicifuga minor back pain
Coffea cruda sleeplessness
Drosera cough
Eupatorium perf flu-like symptoms
Euphrasia eye irritation
Ferrum phos low fever
Gelsemium lethargy
Hamamelis varicose veins
Hepar sulph calc croupiness
Hydrastis sinuses
Hypericum shooting pain
Ignatia sadness
Ipecac nausea
Kali bic sinuses
Kali carb sour belching
Kali mur congested ears
Kali phos irritability
Lachesis sore throat
Ledum black eyes
Lycopodium digestion
Mag phos cramps
Nat mur sneezing
Nux vom vomiting
Petroleum nausea
Phosphoricum ac headache
Phytolacca sore throat
Podoph pelt diarrhea
Pulsatilla weeping
Rhus tox better motion
Ruta bruised feeling
Sepia indifference
Staphysag anger
Sulphur skin problems
Symphytum prickling pain
Thuja occ warts
STOP USE AND ASK DOCTOR
If symptoms persist/worsen or if pregnant or nursing, stop use and consult your practitioner.
DIRECTIONS
Adults: Dissolve 3 to 5 under the tongue three times a day or as directed by a licensed practitioner. Take at greater intervals as condition subsides.
Children: Dissolve 3 to 5 under the tongue three times a day or as directed by a licensed practitioner. Take at greater intervals as condition subsides.