DESCRIPTION
Flurbiprofen sodium ophthalmic solution USP, 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use.
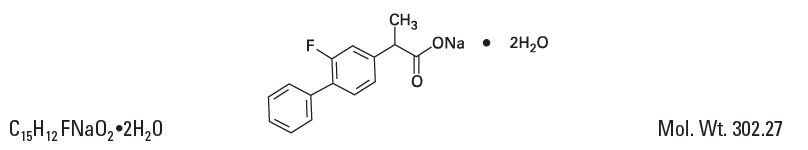
Chemical Name:
Sodium (±)-2-(2-fluoro-4-biphenylyl)propionate dihydrate.
Structural Formula:
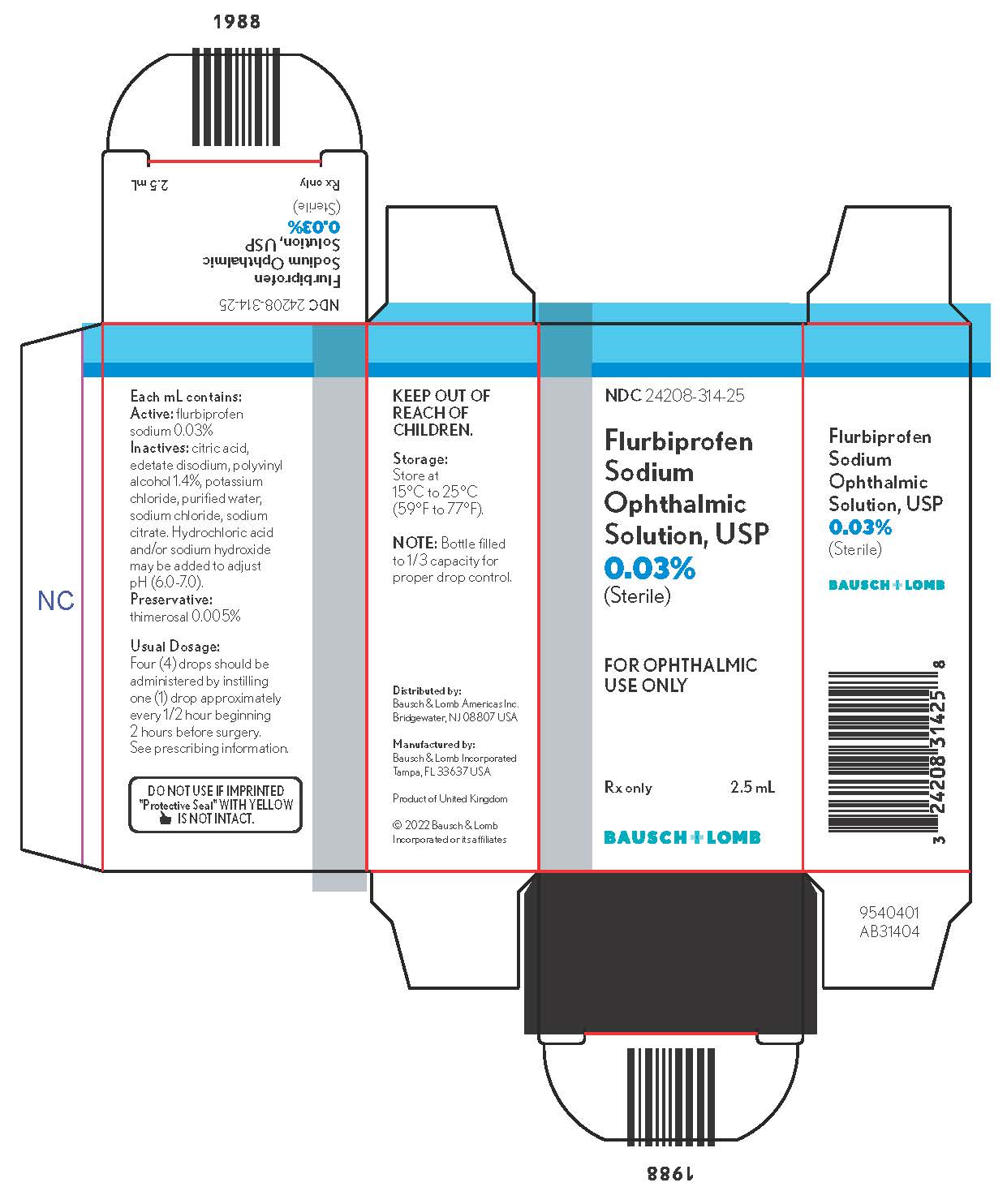
Each mL contains:
Active: flurbiprofen sodium 0.03%.
Inactives: citric acid, edetate disodium, polyvinyl alcohol 1.4%, potassium chloride, purified water, sodium chloride, sodium citrate. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (6.0 – 7.0).
Preservative: thimerosal 0.005%.
CLINICAL PHARMACOLOGY
Flurbiprofen sodium is one of a series of phenylalkanoic acids that have shown analgesic, antipyretic, and anti-inflammatory activity in animal inflammatory diseases. Its mechanism of action is believed to be through inhibition of the cyclo-oxygenase enzyme that is essential in the biosynthesis of prostaglandins.
Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed on animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilatation, increased vascular permeability, leukocytosis, and increased intraocular pressure.
Prostaglandins also appear to play a role in the miotic response produced during ocular surgery by constricting the iris sphincter independently of cholinergic mechanisms. In clinical studies, flurbiprofen sodium ophthalmic solution has been shown to inhibit the miosis induced during the course of cataract surgery.
Results from clinical studies indicate that flurbiprofen sodium has no significant effect upon intraocular pressure.
INDICATIONS AND USAGE
Flurbiprofen sodium ophthalmic solution is indicated for the inhibition of intraoperative miosis.
CONTRAINDICATIONS
Flurbiprofen sodium ophthalmic solution is contraindicated in individuals who are hypersensitive to any components of the medication.
WARNINGS
With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that flurbiprofen sodium ophthalmic solution may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
There is the potential for cross-sensitivity to acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
PRECAUTIONS
General
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
It is recommended that flurbiprofen sodium ophthalmic solution be used with caution in surgical patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
Information for Patients
Patients should be instructed to avoid allowing the tip of the bottle to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
To avoid the potential for cross-contamination, the patient should be advised to use one bottle for each eye with bilateral ocular surgery. The use of the same bottle of eye drops for both eyes is not recommended with ocular surgery.
Drug Interactions
Interaction of flurbiprofen sodium ophthalmic solution with other topical ophthalmic medications has not been fully investigated.
Although clinical studies with acetylcholine chloride and animal studies with acetylcholine chloride or carbachol revealed no interference, and there is no known pharmacological basis for an interaction, there have been reports that acetylcholine chloride and carbachol have been ineffective when used in patients treated with flurbiprofen sodium ophthalmic solution.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in mice and/or rats have shown no evidence of carcinogenicity with flurbiprofen.
Long-term mutagenicity studies in animals have not been performed.
Pregnancy
Flurbiprofen has been shown to be embryocidal, delay parturition, prolong gestation, reduce weight, and/or slightly retard growth of fetuses when given to rats in daily oral doses of 0.4 mg/kg (approximately 300 times the human daily topical dose) and above. There are no adequate and well-controlled studies in pregnant women. Flurbiprofen sodium ophthalmic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from flurbiprofen sodium, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of flurbiprofen sodium ophthalmic solution. Other adverse reactions reported with the use of flurbiprofen sodium ophthalmic solution include: fibrosis, hyphema, miosis, mydriasis, and ocular hyperemia.
Increased bleeding tendency of ocular tissues in conjunction with ocular surgery has also been reported [see Warnings].
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Overdosage will not ordinarily cause acute problems. If accidentally ingested, drink fluids to dilute.
DOSAGE AND ADMINISTRATION
A total of four (4) drops of flurbiprofen sodium ophthalmic solution should be administered by instilling one (1) drop approximately every 1/2 hour beginning 2 hours before surgery.
HOW SUPPLIED
Flurbiprofen sodium ophthalmic solution USP, 0.03% is supplied in a plastic bottle with a controlled drop tip in the following size:
2.5 mL - NDC 24208-314-25