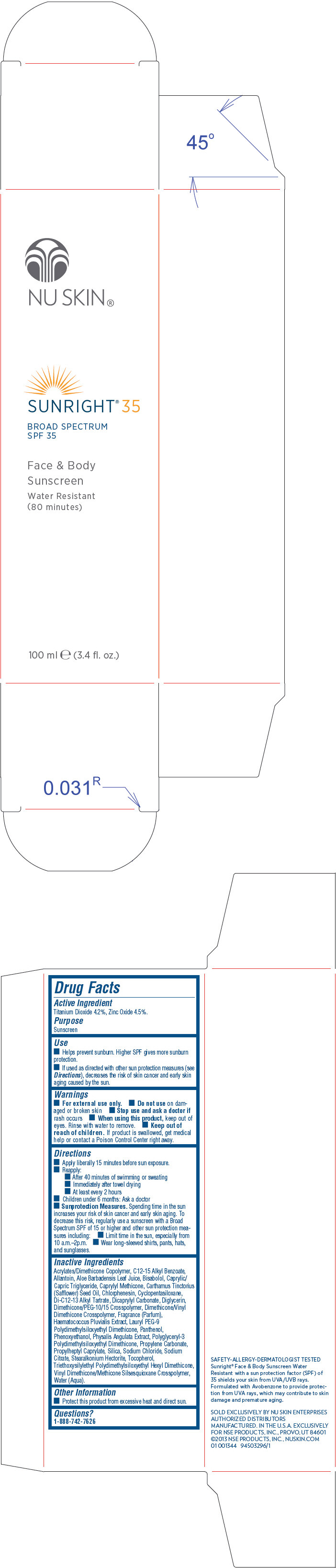
Use
- Helps prevent sunburn. Higher SPF gives more sunburn protection.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply:
- After 40 minutes of swimming or sweating
- Immediately after towel drying
- At least every 2 hours
- Children under 6 months: Ask a doctor
-
Sunprotection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Acrylates/Dimethicone Copolymer, C12-15 Alkyl Benzoate, Allantoin, Aloe Barbadensis Leaf Juice, Bisabolol, Caprylic/Capric Triglyceride, Caprylyl Methicone, Carthamus Tinctorius (Safflower) Seed Oil, Chlorphenesin, Cyclopentasiloxane, Di-C12-13 Alkyl Tartrate, Dicaprylyl Carbonate, Diglycerin, Dimethicone/PEG-10/15 Crosspolymer, Dimethicone/Vinyl Dimethicone Crosspolymer, Fragrance (Parfum), Haematococcus Pluvialis Extract, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Panthenol, Phenoxyethanol, Physalis Angulata Extract, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Propylene Carbonate, Propylheptyl Caprylate, Silica, Sodium Chloride, Sodium Citrate, Stearalkonium Hectorite, Tocopherol, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer, Water (Aqua).