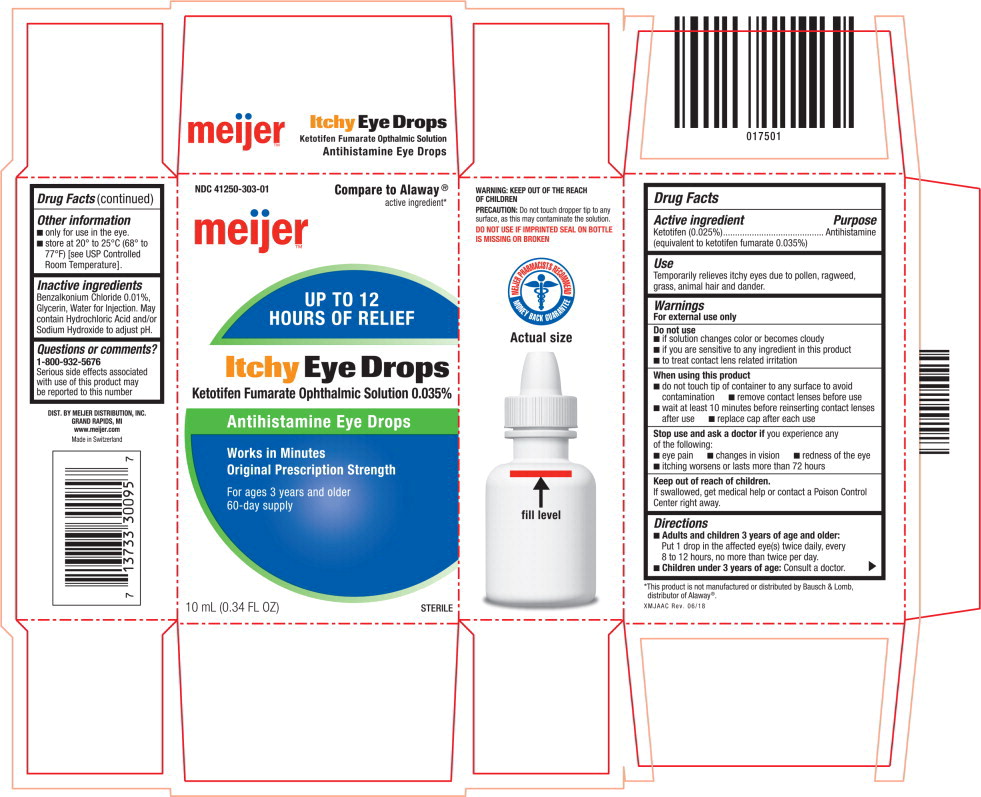
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Directions
- Adults and children 3 years of age and older: Put 1 drop in the affected eye(s) twice daily, every 8 to 12 hours, no more than twice per day.
- Children under 3 years of age: Consult a doctor.
Other information
- only for use in the eye.
- store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Inactive ingredients
Benzalkonium Chloride 0.01%, Glycerin, Water for Injection. May contain Hydrochloric Acid and/or Sodium Hydroxide to adjust pH.
Questions or comments?
1-800-932-5676
Serious side effects associated with use of this product may be reported to this number
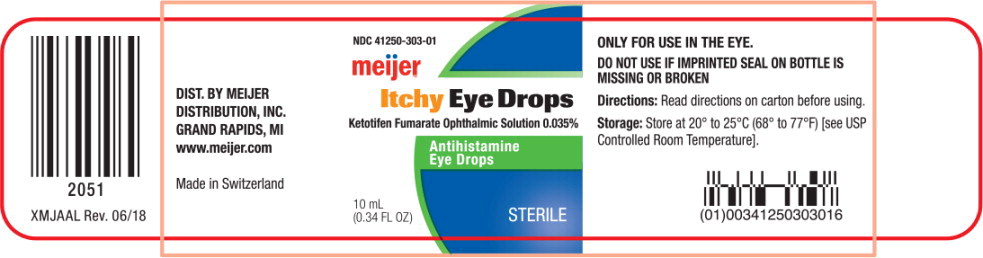
Principal Display Panel Text for Container Label:
NDC 41250-303-01
Meijer™ Logo
Itchy Eye Drops
Ketotifen Fumarate Ophthalmic Solution 0.035%
Antihistamine
Eye Drops
10 mL
0.34 FL OZ
STERILE
Principal Display Panel Text for Carton Label:
NDC 41250-303-01 Compare to Alaway®
active ingredient*
Meijer™ Logo
UP TO 12
HOURS OF RELIEF
Itchy Eye Drops
Ketotifen Fumarate Ophthalmic Solution 0.035%
Antihistamine Eye Drops
Works in Minutes
Original Prescription Strength
For ages 3 years and older
60-day supply
10 mL 0.34 FL OZ STERILE