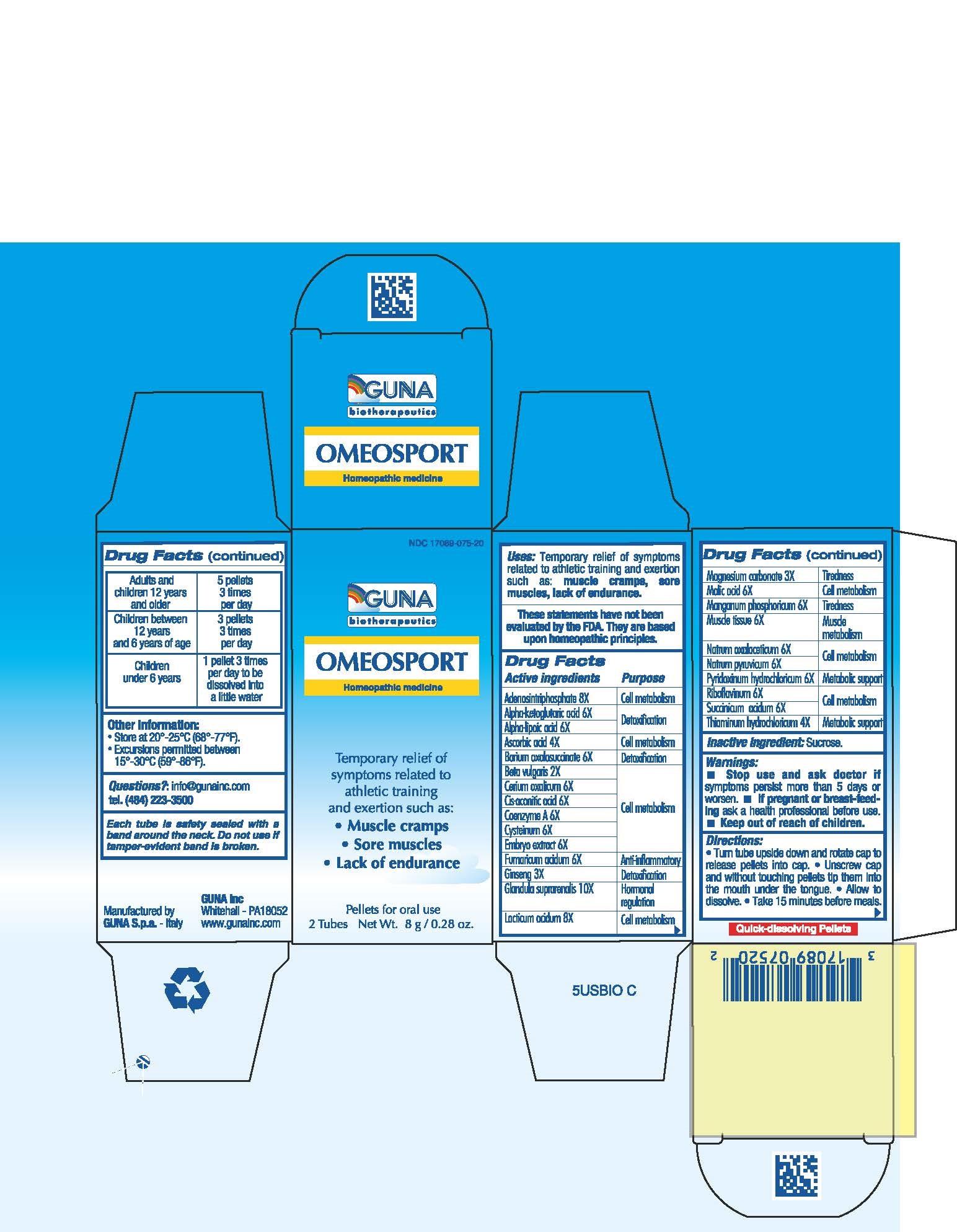
ACTIVE INGREDIENTS/PURPOSE
ADENOSINTRIPHOSPHATE 8X CELL METABOLISM
ALPHA-KETOGLUTARIC ACID 6X DETOXIFICATION
ALPHA-LIPOIC AC 6X DETOXIFICATION
ASCORBIC ACID 4X CELL METABOLISM
BARIUM OXALOSUCCINATE 6X DETOXIFICATION
BETA VULGARIS 2X CELL METABOLISM
CERIUM OXALICUM 6X CELL METABOLISM
CIS-ACONITIC ACID 6X CELL METABOLISM
COENZYME A 6X CELL METABOLISM
CYSTEINUM 6X CELL METABOLISM
EMBRYO EXTRACT 6X CELL METABOLISM
FUMARICUM ACIDUM 6X ANTI-INFLAMMATORY
GINSENG 3X DETOXIFICATION
GLANDULA SUPRARENALIS 10X HORMONAL REGULATION
LACTICUM ACIDUM 8X CELL METABOLISM
MAGNESIUM CARBONATE 2X TIREDNESS
MALIC ACID 6X CELL METABOLISM
MANGANUM PHOSPHORICUM 6X TIREDNESS
MUSCLE TISSUE 6X MUSCLE METABOLISM
NATRUM OXALACETICUM 6X CELL METABOLISM
NATRUM PYRUVICUM 6X CELL METABOLISM
PYRIDOXINUM HYDROCHLORICUM 6X METABOLIC SUPPORT
RIBOFLAVINUM 6X CELL METABOLISM
SUCCINICUM ACIDUM 6X CELL METABOLISM
THIAMINUM HYDROCHLORICUM 4X METABOLIC SUPPORT
USES
For the temporary relief of symptoms related to athletic training and exertion such as:
- Muscle cramps
- Sore muscles
-
Lack of endurance
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days or worsen.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children.
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 5 pellets 3 times per day
Children between 12 years and 6 years of age 3 pellets 3 times per day
Children under 6 years 1 pellet 3 times per day to be dissolved into a little water