LANOLIN- lanolin ointment
Sion Biotext Medical Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
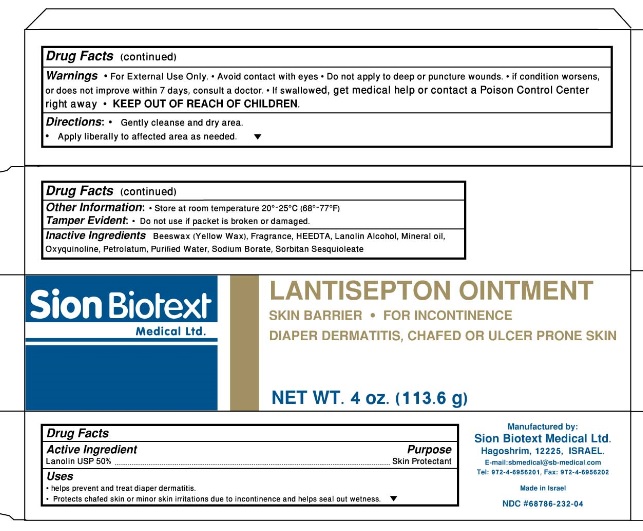
Lantisepton Ointment
Uses:
- Helps prevent and treat diaper dermatitis
- Protects chafed skin or minor skin irritations due to incontinence and helps seal out wetness
Indications and Usage
- Avoid contact with eyes
- Do not apply to deep or puncture wounds
- If condition worsens, or does not improve within 7 days, consult a doctor
- If swallowed, get medical help or contact a Poison Control Center right away
| LANOLIN
lanolin ointment |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sion Biotext Medical Ltd (532775194) |
| Registrant - Sion Biotext Medical Ltd (532775194) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sion Biotext Medical Ltd | 532775194 | manufacture(68786-232) | |
Revised: 12/2022
Document Id: efe1a0f9-ed16-54d7-e053-2a95a90afb80
Set id: 115af0c8-a6fa-4d8e-9b22-9912d7fbc9ba
Version: 4
Effective Time: 20221215
Sion Biotext Medical Ltd