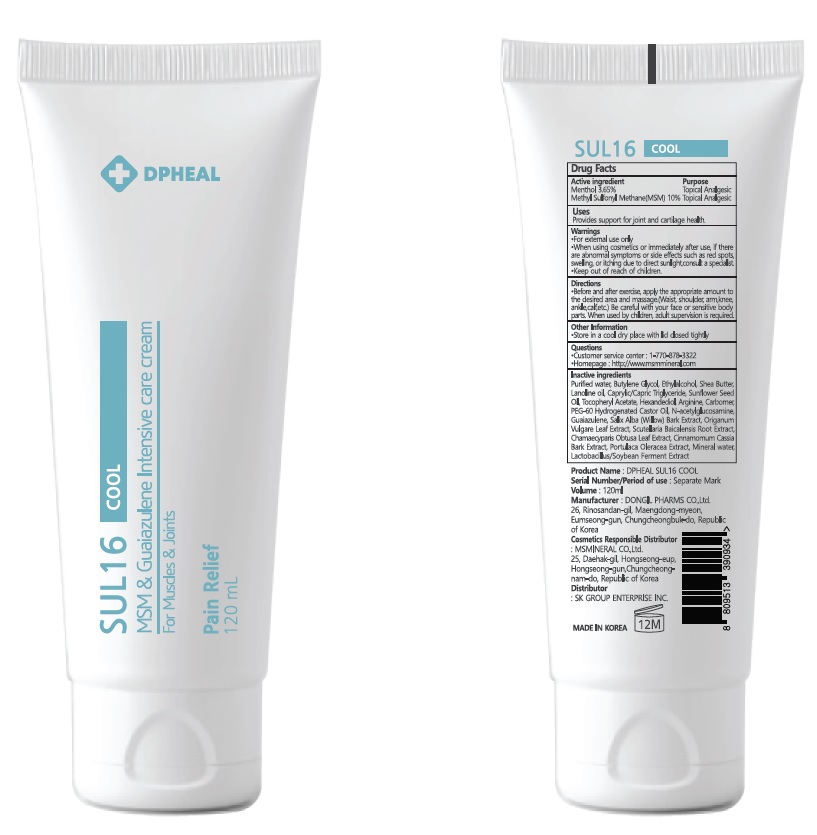
ACTIVE INGREDIENTS
Menthol 3.65%
Methyl Sulfonyl Methane(MSM) 10%
INACTIVE INGREDIENTS
Purified water, Butylene Glycol, Ethylalcohol, Shea Butter, Lanoline oil, Caprylic/Capric Triglyceride, Sunflower Seed Oil, Tocopheryl Acetate, Hexandediol, Arginine, Carbomer, PEG-60 Hydrogenated Castor Oil, N-acetylglucosamine, Guaiazulene, Salix Alba (Willow) Bark Extract, Origanum Vulgare Leaf Extract, Scutellaria Baicalensis Root Extract, Chamaecyparis Obtusa Leaf Extract, Cinnamomum Cassia Bark Extract, Portulaca Oleracea Extract, Mineral water, Lactobacillus/Soybean Ferment Extract
PURPOSE
Topical Analgesic
Uses
■ Provides support for joint and cartilage health.
WARNINGS
■ For external use only
■ When using cosmetics or immediately after use, if there are abnormal symptoms or side effects such as red spots, swelling, or itching due to direct sunlight, consult a specialist.
■ Keep out of reach of children
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
Directions
■ Before and after exercise, apply the appropriate amount to the desired area and massage.
(Waist, shoulder, arm, knee, ankle, calf, etc.) Be careful with your face or sensitive body parts.When used by children, adult supervision is required.
Other Information
■ Store in a cool dry place with lid closed tightly
Questions
■ Customer service center : 1-770-878-3322
■ Homepage : http://www.msmmineral.com
DPHEAL SUL 16 COOL 120mL
NDC: 82409-010-01
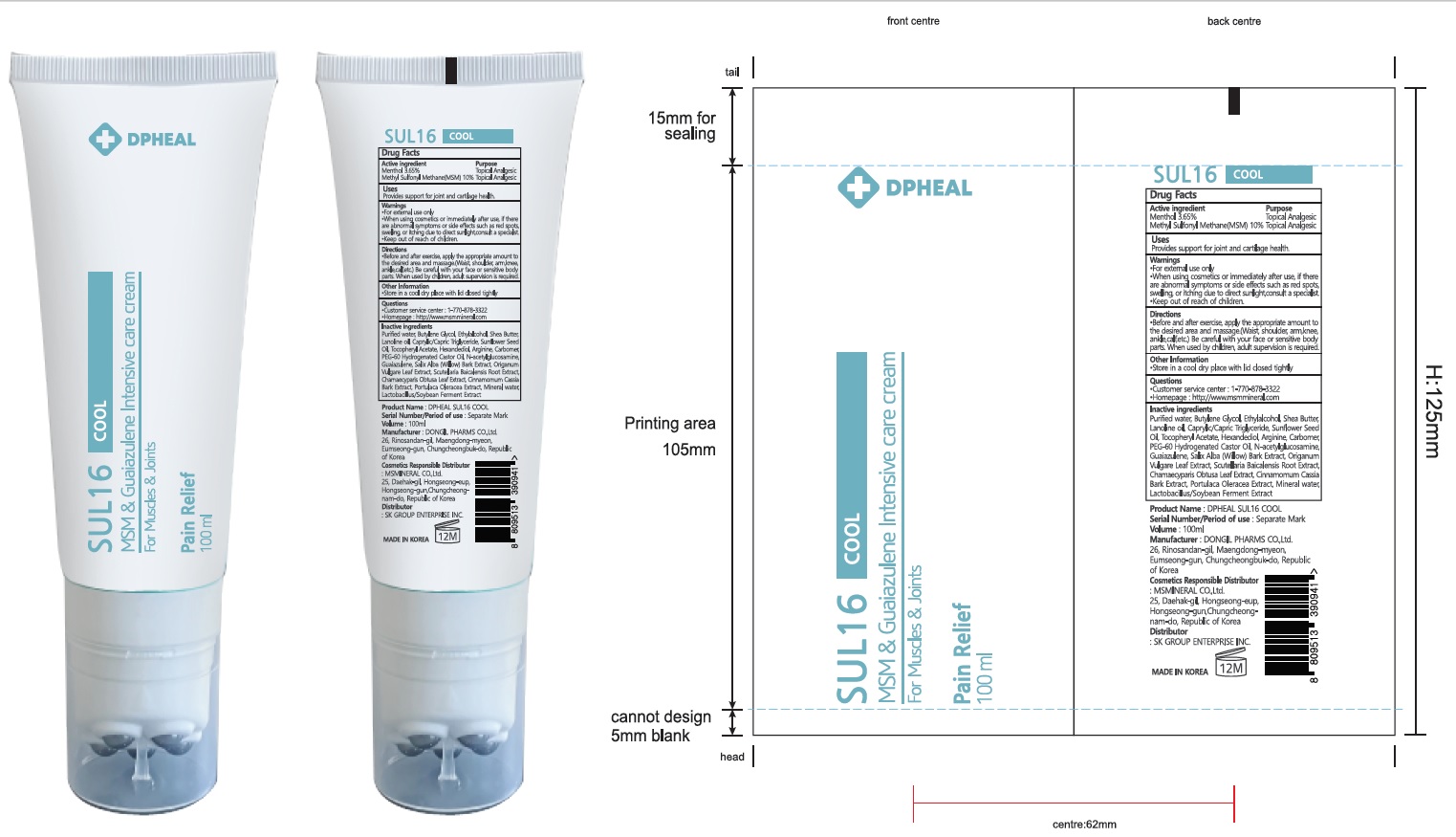
DPHEAL SUL 16 COOL 100mL
NDC: 82409-010-02
DPHEAL SUL 16 COOL 50mL
NDC: 82409-010-03

MSMINERAL CO.,Ltd.


