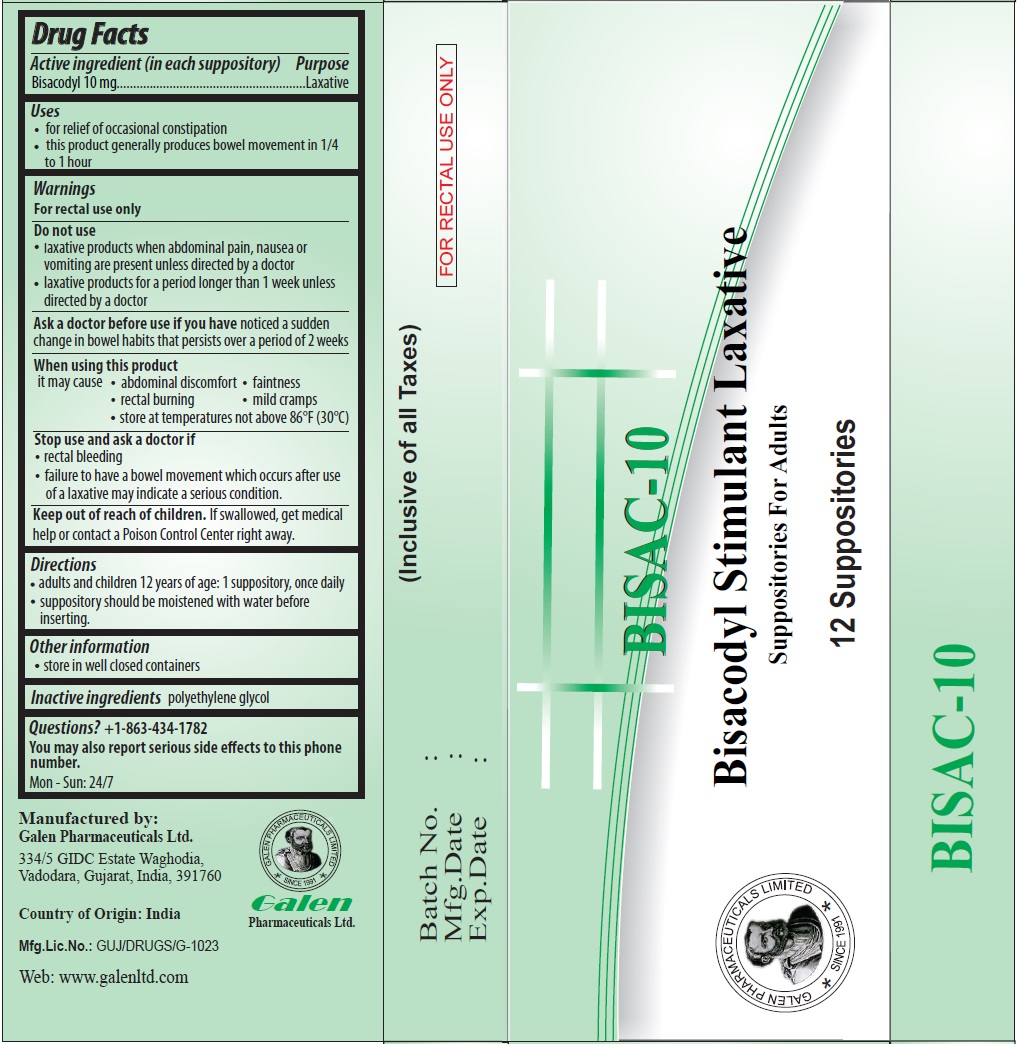
Uses
• for relief of occasional constipation
• this product generally produces bowel movement in 1/4 to 1 hour
Warnings
For rectal use only
Do not use
• laxative products when abdominal pain, nausea or vomiting are present unless directed by a doctor
• laxative products for a period longer than 1 week unless directed by a doctor
Ask a doctor before use if you have noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
it may cause • abdominal discomfort • faintness
• rectal burning • mild cramps
• store at temperatures not above 86°F (30°C)
Stop use and ask a doctor if
• rectal bleeding
• failure to have a bowel movement which occurs after use of a laxative may indicate a serious condition.
Directions
• adults and children 12 years of age: 1 suppository, once daily
• suppository should be moistened with water before inserting.
Questions? +1-863-434-1782
You may also report serious side effects to this phone number.
Mon - Sun: 24/7