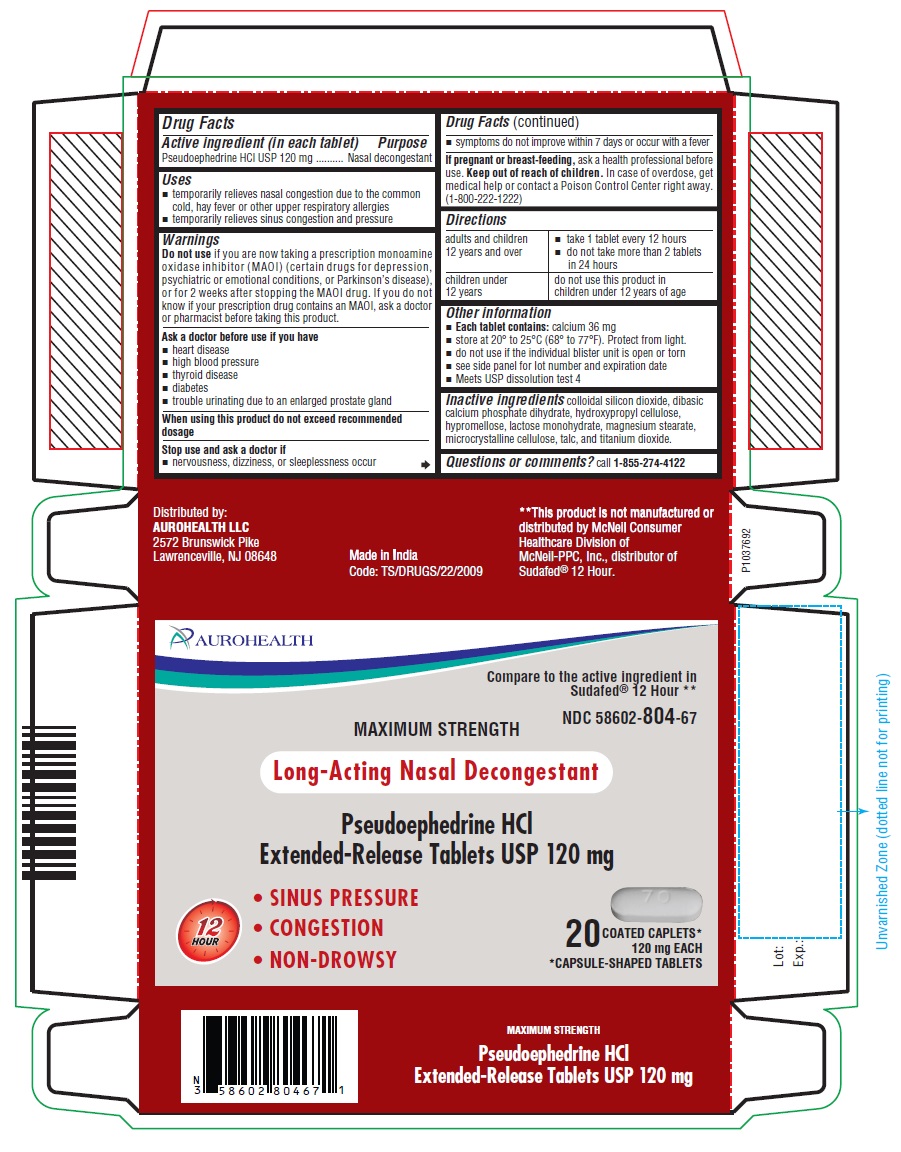
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not improve within 7 days or occur with a fever
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
| adults and children 12 years and over |
|
| children under 12 years
| do not use this product in children under 12 years of age |
Other information
- Each tablet contains: calcium 36 mg
- store at 20° to 25°C (68° to 77°F). Protect from light.
- do not use if the individual blister unit is open or torn
- see side panel for lot number and expiration date
- Meets USP dissolution test 4
Inactive ingredients
colloidal silicon dioxide, dibasic calcium phosphate dihydrate, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, talc, and titanium dioxide.
Questions or comments?
call 1-855-274-4122
Distributed by:
AUROHEALTH LLC
2572 Brunswick Pike
Lawrenceville, NJ 08648
Made in India
Code: TS/DRUGS/22/2009
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 120 mg, Blister Carton 20 (2 X 10) Extended-Release Tablets
AUROHEALTH
Compare to the active ingredient in
Sudafed® 12 Hour**
NDC 58602-804-67
MAXIMUM STRENGTH
Long-Acting Nasal Decongestant
Pseudoephedrine HCl
Extended-Release Tablets USP 120 mg
• SINUS PRESSURE
• CONGESTION
• NON-DROWSY
12 HOUR
20 COATED CAPLETS*
120 mg EACH
*CAPSULE-SHAPED TABLETS