Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Directions
- Adults and children 3 years of age and older: Put 1 drop in the affected eye(s) twice daily, every 8 to 12 hours, no more than twice per day.
- Children under 3 years of age: Consult a doctor.
Other information
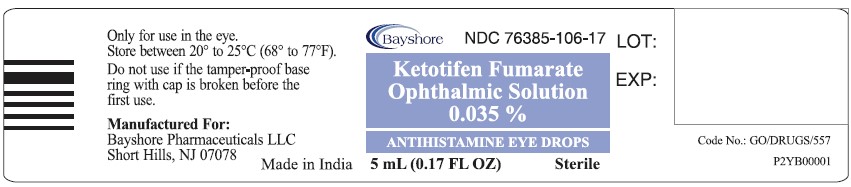
- Only for use in the eye.
- Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Inactive ingredients
benzalkonium chloride 0.01%, glycerol, water for injection, sodium hydroxide and/or hydrochloric acid.
Questions?
Call Product Information at 973-315-1818. Serious side effects associated with use of this product may be reported to this number.
Code No.: GO/DRUGS/557
Manufactured For:
Bayshore Pharmaceuticals
LLC., Short Hills, NJ 07078
Made in India.