UNDA 11- asparagus officinalis young shoot, mentha piperita whole plant, millefolium whole plant,thymus serpyllum whole plant, crocus sativus dried stigma, rosmarinus officinalis flowering twigs, glechoma hederacea whole plant, verbena officinalis whole plant, uva-ursi leaf, cuprum metallicum liquid
Seroyal USA
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients
Each drop contains:
Asparagus officinalis (Asparagus) Shoot 4X
Crocus sativus (Saffron) Dried Stigma 4X
Cuprum metallicum (Copper) 12X
Glechoma hederacea (Ground-ivy) Aerial Parts 4X
Mentha piperita Aerial Parts 4X
Millefolium (Yarrow) Aerial Parts 4X
Rosmarinus officinalis (Rosemary) Leaf 4X
Thymus serpyllum (Wild thyme) Aerial Parts 4X
Uva-ursi (Bearberry) Leaf 4X
Verbena officinalis (Common vervain) Aerial Parts 4X
Uses
For the temporary relief of minor pain
associated with
headaches
joints
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a
Poison Control Center right away.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a
Poison Control Center right away.
Other information
Do not use if seal is missing or broken.
Store in a cool, dry place.
Inactive ingredients
Ethanol (beet), purified water
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of minor pain
associated with
headaches
joints
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
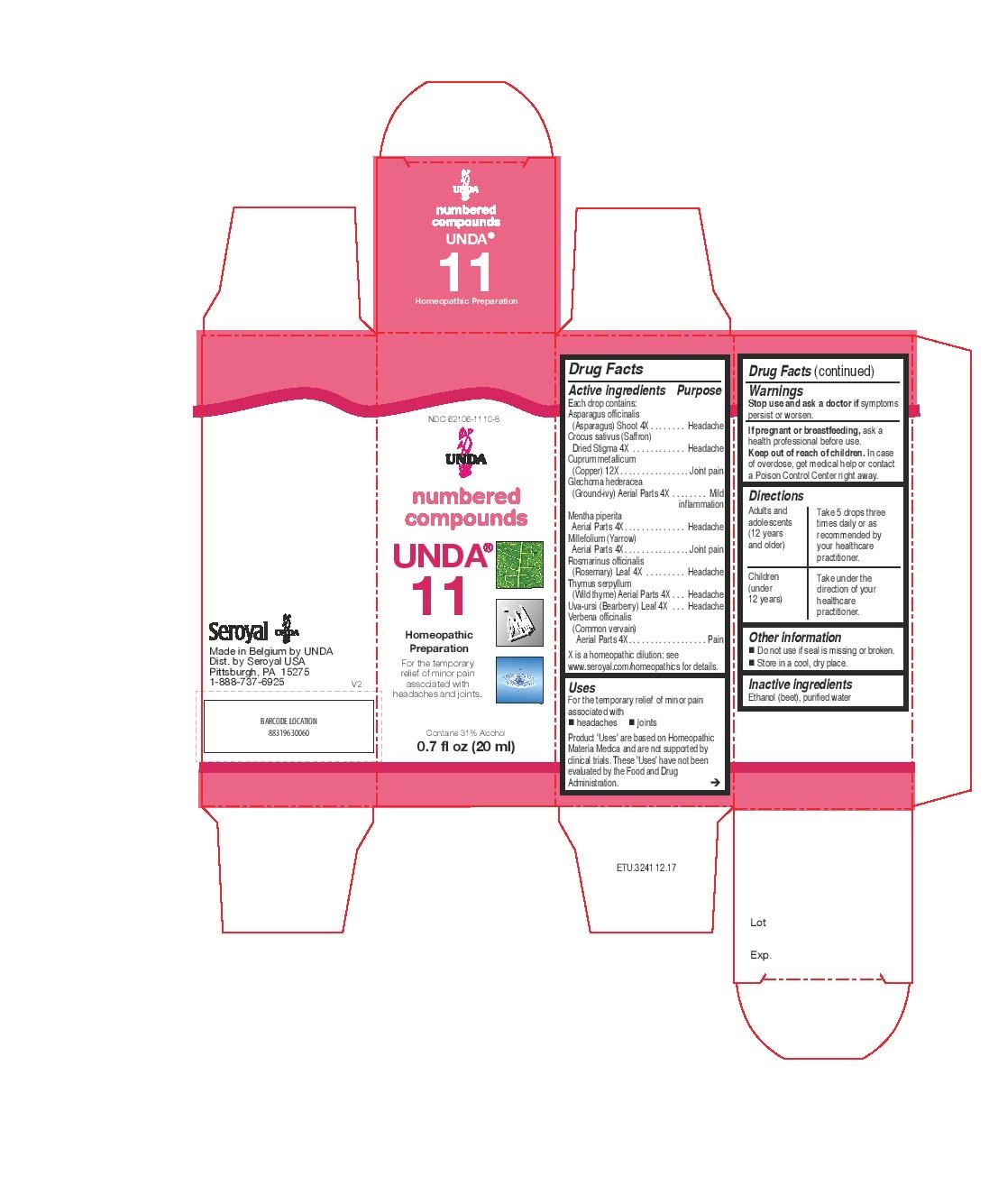
NDC 62106-1110-8
UNDA
numbered compounds
UNDA 11
Homeopathic Preparation
For the temporary relief of minor pain
associated with headaches and joints.
Contains 31% Alcohol
0.7 fl oz (20 ml)
