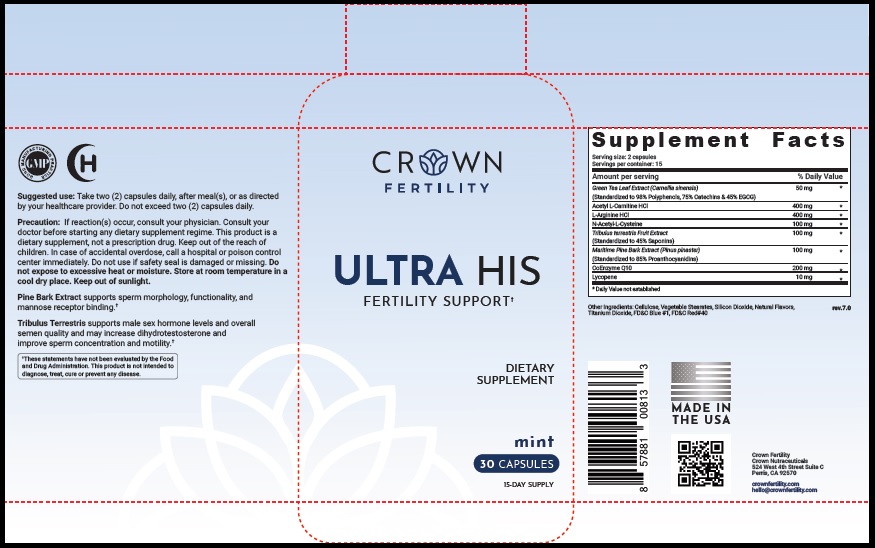
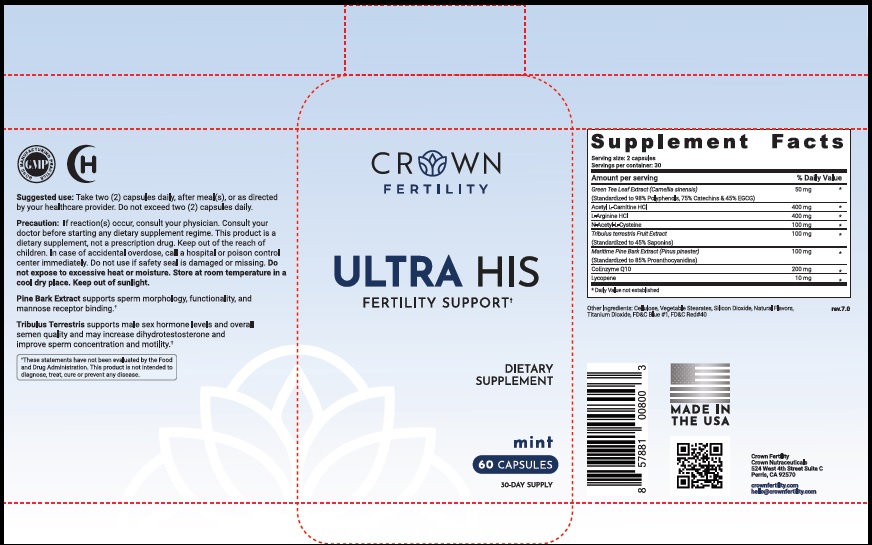
Suggested Use
Take two (2) capsules daily, after meal(s), or as directed by your healthcare provider. Do not exceed two (2) capsules daily.
Precaution
If reaction(s) occur, consult your physician. Consult your doctor before starting any dietary supplement regime. This product is a dietary supplement, not a prescription drug. Keep out of the reach of children. In case of accidental overdose, call a hospital or poison control center immediately. Do not use if safety seal is damaged or missing. Do not expose to excessive heat or moisture. Store at room temperature in a cool dry place. Keep out of sunlight.