Uses
- For the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
- May be used as a protectant against further irritation.
Warnings
-
For external use only.
-
To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
-
Do not touch unit-dose tip to eye.
- If solution changes color or becomes cloudy, do not use.
Directions
To open, TWIST AND PULL TAB TO REMOVE.
Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
*Follow your eye doctor’s instructions if you are using this product after an eye surgery (e.g., LASIK) to relieve eye dryness and discomfort.
Other information
- Use only if single-use container is intact.
- Use before expiration date marked on container.
- Store at 59°-77°F (15°-25°C).
- RETAIN THIS CARTON FOR FUTURE REFERENCE.
Inactive ingredients
Calcium chloride dihydrate; magnesium chloride hexahydrate; potassium chloride; purified water; sodium chloride; and sodium lactate. May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
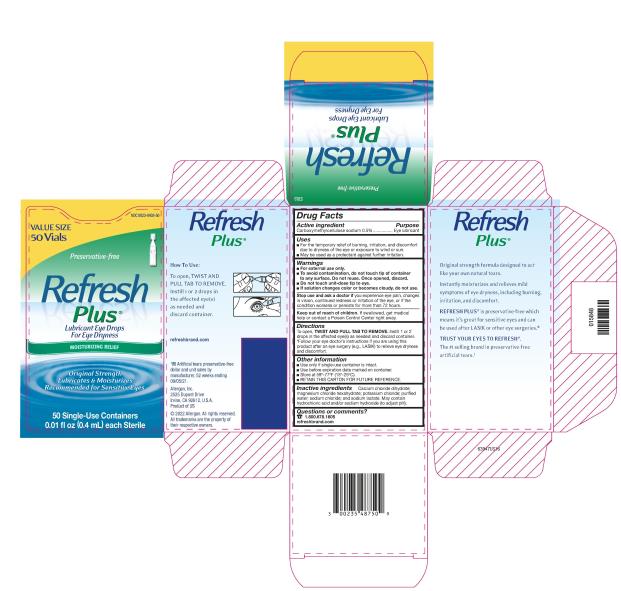
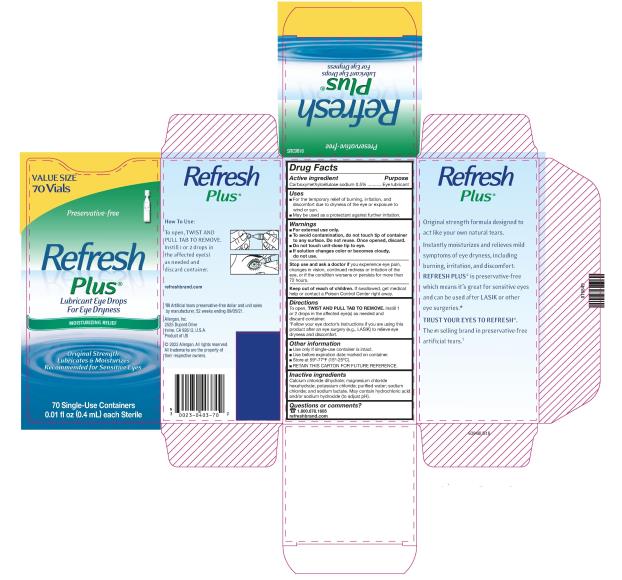
PRINCIPAL DISPLAY PANEL
NDC 0023-0403-30
Preservative-free
Refresh
Plus®
Lubricant Eye Drops
For Eye Dryness
MOISTURIZING RELIEF
Original Strength
Lubricates & Moisturizes
Recommended for Sensitive Eyes
30 Single-Use Containers
0.01 fl oz (0.4 mL) each Sterile