ELIGARD- leuprolide acetate injection, suspension, extended release
TOLMAR Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ELIGARD® safely and effectively. See full prescribing information for ELIGARD. ELIGARD (leuprolide acetate) for injectable suspension, for subcutaneous use Initial U.S. Approval: 2002 RECENT MAJOR CHANGESDosage and Administration (2) 09/2022 INDICATIONS AND USAGEELIGARD is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of advanced prostate cancer. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Tolmar at 1-888-354-4273 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 9/2023 |
FULL PRESCRIBING INFORMATION
2. DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
ELIGARD 45 mg is administered subcutaneously every 6 months and provides continuous release of leuprolide acetate over a six-month treatment period. ELIGARD must be administered by a healthcare provider. The injection delivers the dose of leuprolide acetate incorporated in a polymer formulation.
As with other drugs administered by subcutaneous injection, the injection site should vary periodically. The specific injection location should be an area with sufficient soft or loose subcutaneous tissue. In clinical trials, the injections were administered in the upper- or mid-abdominal area. Avoid areas with brawny or fibrous subcutaneous tissue or locations that could be rubbed or compressed (i.e., with a belt or clothing waistband).
2.2 Mixing Procedure
Use aseptic technique throughout the procedure. As with other similar agents, the use of gloves is recommended during mixing and administration.1 Allow the product to reach room temperature before mixing. Once mixed, the product must be administered within 30 minutes or it should be discarded.
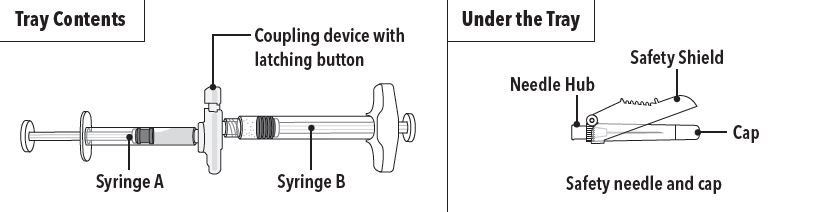
ELIGARD is packaged in a carton containing:
- Tray containing pre-connected syringe system and desiccant pack
- Prescribing information
- Sterile safety needle and cap (located under the tray in carton)
Follow the detailed instructions below to ensure correct preparation of ELIGARD prior to administration:
|
Step 1 On a clean field open the tray by tearing off the foil from the corner and remove the contents. Discard the desiccant pack. Remove the pre-connected syringe system from the tray. Open the sterile safety needle package by peeling back the paper tab. Note: Syringe A and Syringe B should not be lined-up yet. The product should only be administered with the co-packaged, sterile safety needle. |
|
 |
|
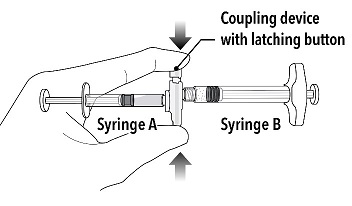
| Step 2
Grasp the latching button on the coupling device with your finger and thumb and press until you hear a snapping sound. The two syringes will be aligned.
|  |
| Step 3 Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprolide acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by pushing the contents back and forth between both syringes to obtain a uniform suspension.
Note: Product must be mixed as described; shaking will NOT provide adequate mixing. Do not bend. | 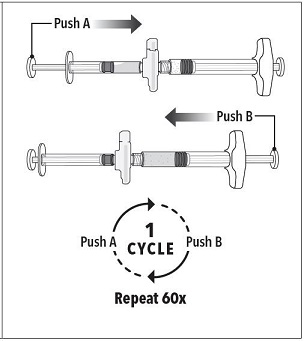 |
| Step 4 After mixing, hold the syringes vertically (upright) with Syringe B (wide syringe) on the bottom. The syringes should remain securely coupled. Transfer all of the mixed product into Syringe B by depressing the Syringe A plunger and slightly withdrawing the Syringe B plunger. | 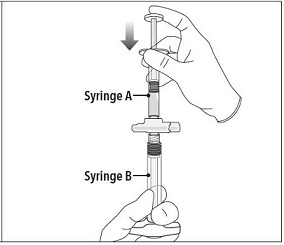 |
| Step 5 While ensuring the Syringe A plunger is fully pushed down, hold the coupling device and unscrew Syringe B. This will disconnect Syringe B from the coupling device. Syringe A will remain attached to the coupling device.
Note: Small air bubbles will remain in the formulation – this is acceptable.
Do not purge the air bubbles from Syringe B as product may be lost! | 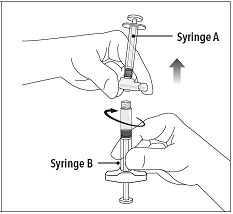 |
| Step 6 Continue to hold Syringe B upright with the open end at the top. Hold back the white plunger on Syringe B to prevent loss of the product and attach the safety needle and cap. Gently screw clockwise with approximately a three-quarter turn until the safety needle and cap are secure.
Do not overtighten, as the needle hub may become damaged which could result in leakage of the product during injection. The safety shield may also be damaged if the safety needle and cap are screwed with too much force. | 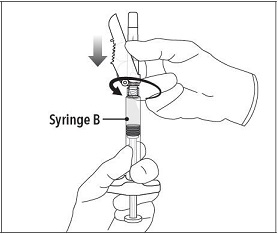 |
| Step 7 Move the safety shield away from the needle and towards the syringe. Pull off the cap immediately prior to administration. | 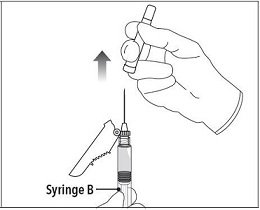 |
Note: Should the needle hub appear to be damaged, or leak, the product should NOT be used. The damaged safety needle and cap should NOT be replaced and the product should NOT be injected. In the event of damage to the needle hub, use a new replacement ELIGARD carton.
2.3 Administration Procedure
|
1. Select an injection site on the abdomen, upper buttocks, or another location with adequate amounts of subcutaneous tissue that does not have excessive pigment, nodules, lesions, or hair and hasn’t recently been used. 2. Cleanse the injection-site area with an alcohol swab (not enclosed). 3. Using the thumb and forefinger, grab and bunch the area of skin around the injection site. | 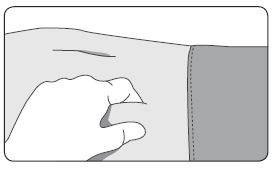 |
| 4. Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and fullness of the subcutaneous tissue and the length of the needle. After the needle is inserted, release the skin. 5. Inject the drug using a slow, steady push and press down on the plunger until the syringe is empty. Make sure all the drug has been injected before removing the needle. 6. Withdraw the needle quickly at the same 90° angle used for insertion. | 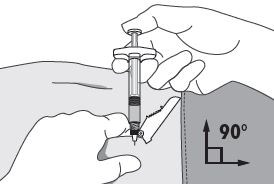 |
|
7. Immediately following the withdrawal of the needle, activate the safety shield using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place. 8. An audible and tactile “click” verifies a locked position. 9. Check to confirm the safety shield is fully engaged. Discard all components safely in an appropriate biohazard container. | 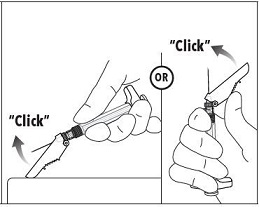 |
3. DOSAGE FORMS AND STRENGTHS
ELIGARD is an injectable suspension of leuprolide acetate available in a pre-connected syringe system and packaged with a sterile safety needle and cap (Table 1), a desiccant, prescribing information and instructions for use. The pre-connected syringe system consists of syringe A and syringe B connected using a coupling device. Syringe A contains the in situ polymeric extended release technology and the syringe B contains leuprolide acetate powder. When reconstituted, ELIGARD is administered as a single dose.
Table 1. Specifications for ELIGARD Sterile Safety Needle and Cap
| ELIGARD® strength | Gauge | Length |
| 45 mg | 18-gauge | 5/8-inch |
4. CONTRAINDICATIONS
Hypersensitivity
ELIGARD is contraindicated in patients with hypersensitivity to GnRH, GnRH agonist analogs or any of the components of ELIGARD. Anaphylactic reactions to synthetic GnRH or GnRH agonist analogs have been reported in the literature.
5. WARNINGS AND PRECAUTIONS
5.1 Tumor Flare
ELIGARD 45 mg causes a transient increase in serum concentrations of testosterone during the first two weeks of treatment. Patients may experience worsening of symptoms or onset of new signs and symptoms during the first few weeks of treatment, including bone pain, neuropathy, hematuria, or bladder outlet obstruction.
Cases of ureteral obstruction and/or spinal cord compression, which may contribute to paralysis with or without fatal complications, have been observed in the palliative treatment of advanced prostate cancer using GnRH agonists.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy. If spinal cord compression or ureteral obstruction develops, standard treatment of these complications should be instituted.
5.2 Laboratory Tests
Monitor ELIGARD response by periodic measurement of serum concentrations of testosterone and prostate specific antigen.
In the majority of patients, testosterone levels increased above Baseline during the first week, declining thereafter to Baseline levels or below by the end of the second or third week. Castrate levels were generally reached within two to four weeks.
Once castrate levels were achieved with ELIGARD 45 mg, only one patient (< 1%) experienced a breakthrough, with testosterone levels > 50 ng/dL.
Results of testosterone determinations are dependent on assay methodology. It is advisable to be aware of the type and precision of the assay methodology to make appropriate clinical and therapeutic decisions.
Drug/Laboratory Test Interactions: Therapy with leuprolide acetate results in suppression of the pituitary-gonadal system. Results of diagnostic tests of pituitary gonadotropic and gonadal functions conducted during and after leuprolide therapy may be affected.
5.3 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
5.4 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
5.5 Effect on QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.6 Embryo-Fetal Toxicity
Based on findings in animal studies and mechanism of action, leuprolide acetate may cause fetal harm when administered to a pregnant woman. In animal developmental and reproductive studies, major fetal abnormalities were observed after administration of leuprolide acetate throughout gestation in rats. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1), Clinical Pharmacology (12.1)].
5.7 Convulsions
Postmarketing reports of convulsions have been observed in patients on leuprolide acetate therapy. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above. Patients receiving a GnRH agonist who experience convulsion should be managed according to current clinical practice.
6. ADVERSE REACTIONS
6.1 Clinical Trial Experience
The safety of ELIGARD was evaluated in clinical trials involving patients with advanced prostate cancer (Table 3). ELIGARD, like other GnRH analogs, caused a transient increase in serum testosterone concentrations during the first one to two weeks of treatment. Therefore, potential exacerbations of signs and symptoms of the disease during the first weeks of treatment are of concern in patients with vertebral metastases and/or urinary obstruction or hematuria. If these conditions are aggravated, it may lead to neurological problems such as weakness and/or paresthesia of the lower limbs or worsening of urinary symptoms [see WARNINGS AND PRECAUTIONS (5.2)].
During the clinical trials, injection sites were closely monitored. Refer to Table 2 for a summary of reported injection site adverse reactions.
Table 2. Reported Injection Site Adverse Reactions
|
ELIGARD 45 mg |
|
|
Study number |
AGL0205 |
|
Number of patients |
111 |
|
Treatment |
1 injection every 6 months up to 12 months |
|
Number of injections |
217 |
|
Transient burning/stinging |
35 (16%) injections; 91.4% reported as mild |
|
Pain (generally brief and mild) |
4.6% of injections1 |
| Bruising (mild) | 2.3% of injections2 |
|
|
These localized adverse reactions were non-recurrent over time. No patient discontinued therapy due to an injection site adverse reaction.
The following possibly or probably related systemic adverse reactions occurred during clinical trials with ELIGARD, and were reported in > 2% of patients (Table 3). Often, causality is difficult to assess in patients with metastatic prostate cancer. Reactions considered not drug-related are excluded.
Table 3. Summary of Possible or Probably Related Systemic Adverse Reactions Reported by > 2% of Patients Treated with ELIGARD
|
ELIGARD 45 mg |
|
||||
|
Study number |
AGL0205 |
||||
|
Number of patients |
111 |
||||
|
Treatment |
1 injection every 6 months up to 12 months |
||||
| Body system | Adverse Reaction | ||||
| Body as a whole | Malaise and fatigue | 13 (11.7%) | |||
| Weakness | 4 (3.6%) | ||||
| Vascular | Hot flashes/sweats | 64 (57.7%)* | |||
| Skin | Night sweats | 3 (2.7%)* | |||
| Musculoskeletal | Myalgia | 5 (4.5%) | |||
| Pain in limb | 3 (2.7%) | ||||
| Reproductive | Testicular atrophy | 8 (7.2%)* | |||
| Gynecomastia | 4 (3.6%)* | ||||
|
*Expected pharmacological consequences of testosterone suppression. In the patient population studied with ELIGARD 45 mg, a total of 89 hot flash adverse reactions were reported in 64 patients. Of these, 62 adverse reactions (70%) were mild; 27 (30%) were moderate; none were severe. |
|||||
In addition, the following possibly or probably related systemic adverse reactions were reported by < 2% of the patients treated with ELIGARD in clinical studies.
|
Body system |
Adverse Reactions |
|
General |
Sweating, insomnia, syncope, rigors, weakness, lethargy |
|
Gastrointestinal |
Flatulence, constipation, dyspepsia |
|
Hematologic |
Decreased red blood cell count, hematocrit and hemoglobin |
|
Metabolic |
Weight gain |
|
Musculoskeletal |
Tremor, backache, joint pain, muscle atrophy, limb pain |
|
Nervous |
Disturbance of smell and taste, depression, vertigo |
|
Psychiatric |
Insomnia, depression, loss of libido* |
|
Renal/urinary |
Difficulties with urination, pain on urination, scanty urination, bladder spasm, blood in urine, urinary retention, urinary urgency, incontinence, nocturia, nocturia aggravated |
|
Reproductive/ |
Testicular soreness/pain, impotence*, decreased libido*, gynecomastia*, breast soreness/tenderness*, testicular atrophy*, erectile dysfunction, penile disorder*, reduced penis size |
|
Skin |
Alopecia, clamminess, night sweats*, sweating increased* |
|
Vascular |
Hypertension, hypotension |
|
* Expected pharmacological consequences of testosterone suppression. |
|
Changes in Bone Density: Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with a GnRH agonist analog. It can be anticipated that long periods of medical castration in men will have effects on bone density.
6.2 Postmarketing Experience
Pituitary apoplexy-During post-marketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
Nervous System-Convulsions
Respiratory System-Interstitial lung disease
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, ELIGARD may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. Expected hormonal changes that occur with ELIGARD treatment increase the risk for pregnancy loss. In animal developmental and reproductive studies, major fetal abnormalities were observed after administration of leuprolide acetate throughout gestation in rats. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus (see Data).
Animal Data
In animal developmental and reproductive studies, major fetal abnormalities were observed after administration of leuprolide acetate throughout gestation. There were increased fetal mortality and decreased fetal weights in rats and rabbits. The effects of fetal mortality are expected consequences of the alterations in hormonal levels brought about by this drug.
8.2 Lactation
The safety and efficacy of ELIGARD have not been established in females. There is no information regarding the presence of ELIGARD in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in a breastfed child from ELIGARD, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Based on mechanism of action, ELIGARD may impair fertility in males of reproductive potential [see Clinical Pharmacology (12.1)].
10. OVERDOSAGE
In clinical trials using daily subcutaneous injections of leuprolide acetate in patients with prostate cancer, doses as high as 20 mg/day for up to two years caused no adverse effects differing from those observed with the 1 mg/day dose.
11. DESCRIPTION
ELIGARD 45 mg is a sterile polymeric matrix formulation of leuprolide acetate, a GnRH agonist, for subcutaneous injection. It is designed to deliver leuprolide acetate at a controlled rate over a six-month therapeutic period.
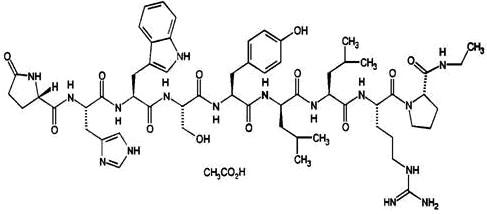
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone (GnRH) that, when given continuously, inhibits pituitary gonadotropin secretion and suppresses testicular and ovarian steroidogenesis. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
ELIGARD is supplied as a pre-connected syringe system comprised of two prefilled syringes (syringe A and syringe B) connected using a coupling device. Immediately prior to administration, the contents of the pre-connected syringe system are mixed until homogenous. ELIGARD is administered subcutaneously, where it forms a solid drug delivery depot.
Syringe A contains the in situ polymeric extended release technology consisting of a biodegradable poly (DL-lactide-co-glycolide) (PLG) polymer formulation dissolved in a biocompatible solvent, N-methyl-2-pyrrolidone (NMP) and syringe B contains leuprolide acetate.
Refer to Table 4 for the delivery system composition and reconstituted product formulation for the ELIGARD product.
Table 4. ELIGARD Delivery System Composition and Reconstituted Product Formulation
|
ELIGARD 45 mg |
|
|
|
In situ polymeric extended release technology |
Polymer |
PLG |
|
Polymer description |
Copolymer with hexanediol |
|
|
Polymer DL-lactide to glycolide |
85:15 |
|
|
Reconstituted product
|
Polymer delivered |
165 mg |
|
NMP delivered |
165 mg |
|
|
Leuprolide acetate delivered |
45 mg |
|
|
Approximate leuprolide free base equivalent |
42 mg |
|
|
Approximate administered formulation weight |
375 mg |
|
|
Approximate injection volume | 0.375 mL | |
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Leuprolide acetate, a gonadotropin releasing hormone (GnRH) agonist, acts as a potent inhibitor of gonadotropin secretion when given continuously in therapeutic doses. Animal and human studies indicate that after an initial stimulation, chronic administration of leuprolide acetate results in suppression of testicular and ovarian steroidogenesis. This effect is reversible upon discontinuation of drug therapy.
In humans, administration of leuprolide acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH), leading to a transient increase in levels of the gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females). However, continuous administration of leuprolide acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to below castrate threshold (≤50 ng/dL). These decreases occur within two to four weeks after initiation of treatment. Long-term studies have shown that continuation of therapy with leuprolide acetate maintains testosterone below the castrate level for up to seven years.
12.2 Pharmacodynamics
Following the first dose of ELIGARD, mean serum testosterone concentrations transiently increased, then fell to below castrate threshold (< 50 ng/dL) within three weeks for all ELIGARD concentrations.
One patient at Day 1 and another patient at Day 29 were withdrawn from the ELIGARD 45 mg study. Of the 109 patients remaining in the study, 108 (99.1%) had serum testosterone levels below the castrate threshold by Month 1 (Day 28). One patient did not achieve castrate suppression and was withdrawn from the study at Day 85. Once castrate testosterone suppression was achieved, one patient (< 1%) demonstrated breakthrough (concentrations > 50 ng/dL after achieving castrate levels) (Figure 1).
Leuprolide acetate is not active when given orally.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics/pharmacodynamics observed during injections administered initially and at six months (ELIGARD 45 mg) in 27 patients with advanced prostate cancer is shown in Figure 1. Mean serum leuprolide concentrations rose to 82 ng/mL and 102 ng/mL (Cmax) at approximately 4.5 hours following the initial and second injections, respectively. After the initial increase following each injection, mean serum concentrations remained relatively constant (0.2 – 2.0 ng/mL).
Figure 1. Pharmacokinetic/Pharmacodynamic Response (N=27) to ELIGARD 45 mg - Patients Dosed Initially and at Month 6
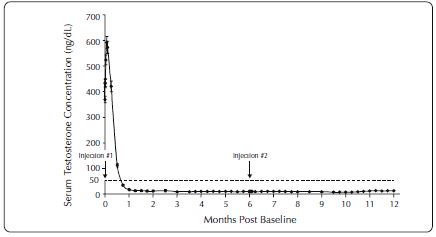
There was no evidence of significant accumulation during repeated dosing. Non-detectable leuprolide plasma concentrations have been occasionally observed during ELIGARD administration, but testosterone levels were maintained at castrate levels.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Elimination
In healthy male volunteers, a 1-mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 8.34 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model.
Metabolism
Upon administration with different leuprolide acetate formulations, the major metabolite of leuprolide acetate is a pentapeptide (M-1) metabolite.
Specific Populations
Geriatrics. [see USE IN SPECIAL POPULATIONS (8.5)]
Race
In patients studied, mean serum leuprolide concentrations were similar regardless of race [White (n = 80), Black (n = 15)] and ethnicity [Hispanic (n=9)].
Renal and Hepatic Impairment
The pharmacokinetics of ELIGARD in hepatically and renally impaired patients have not been determined.
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted with leuprolide acetate in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities. No carcinogenicity studies have been conducted with ELIGARD.
Mutagenicity studies have been performed with leuprolide acetate using bacterial and mammalian systems and with ELIGARD 7.5 mg in bacterial systems. These studies provided no evidence of a mutagenic potential.
14. CLINICAL STUDIES
One open-label, multicenter study (AGL0205) was conducted in patients with Jewett stage A though D prostate cancer who were treated with at least a single injection of 45 mg of ELIGARD (Table 5). This study evaluated the achievement and maintenance of castrate serum testosterone suppression over the duration of therapy (Figure 2).
During the AGL0205 study using ELIGARD 45 mg, once testosterone suppression was achieved, one patient (<1%) demonstrated breakthrough. This patient reached castrate suppression at Day 21 and remained suppressed until Day 308 when his testosterone level rose to 112 ng/dL. At Month 12 (Day 336), his testosterone was 210 ng/dL.
Table 5. Summary of Patients in Study AGL0205
|
ELIGARD 45 mg |
|
||
|
Study number |
AGL0205 |
||
|
Total number of patients |
111 (103 completed1) |
||
|
Jewett stages |
Stage A |
5 |
|
|
Stage B |
43 |
||
|
Stage C |
19 |
||
|
Stage D |
44 |
||
|
Treatment |
1 injection (5 patients) |
||
|
2 injections, one every six months (106 patients) |
|||
|
Duration of therapy |
12 months |
||
|
Mean testosterone concentration (ng/dL) |
Baseline |
367.7 |
|
|
Day 2 |
588.6 |
||
|
Day 14 |
Below Baseline |
||
|
Day 28 |
16.7 |
||
|
Conclusion |
12.6 |
||
|
Number of patients below castrate threshold |
Day 28 |
108 of 109 (99.1%) |
|
|
Conclusion |
102 (99%) |
||
|
|||
Figure 2. ELIGARD 45 mg Mean Serum Testosterone Concentrations (n=103)
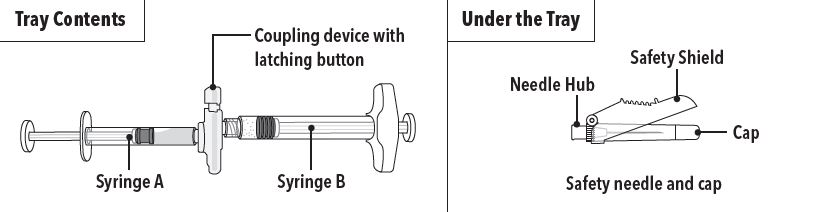
Serum PSA decreased in all patients whose Baseline values were elevated above the normal limit. Refer to Table 6 for a summary of the effectiveness of ELIGARD in reducing serum PSA values.
Table 6. Effect of ELIGARD on Patient Serum PSA Values
|
ELIGARD 45 mg |
|
|
Mean PSA reduction at study conclusion | 97% |
|
Patients with normal PSA at study conclusion* | 95% |
|
*Among patients who presented with elevated levels at Baseline |
|
Other secondary efficacy endpoints evaluated included WHO performance status, bone pain, urinary pain and urinary signs and symptoms. Refer to Table 7 for a summary of these endpoints.
Table 7. Secondary Efficacy Endpoints
| Secondary Efficacy Endpoint | Baseline | Month 12 |
|
WHO Status = 01 |
90% | 94% |
| WHO Status = 12 | 7% | 5% |
| WHO Status = 23 | 3% | 1% |
|
Mean bone pain4
|
1.38 (1-7) | 1.31 (1-8) |
|
Mean urinary pain (range) |
1.22 (1-8) | 1.07 (1-5) |
|
Mean urinary signs and symptoms (range) |
Low | Modestly decreased |
|
Number of patients with prostate abnormalities |
89 (80%) | 60 (58%) |
|
||
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
Hypersensitivity
- Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like ELIGARD, ELIGARD is contraindicated [see Contraindications (4)].
Tumor Flare
- Inform patients that ELIGARD can cause tumor flare during the first weeks of treatment. Inform patients that the increase in testosterone can cause an increase in urinary symptoms or pain. Advise patients to contact their healthcare provider if uretral obstruction, spinal cord compression, paralysis, or new or worsened symptoms occur after beginning ELIGARD treatment [see Warnings and Precautions (5.1)].
Hyperglycemia and Diabetes
- Advise patients that there is an increased risk of hyperglycemia and diabetes with ELIGARD therapy. Inform patients that periodic monitoring for hyperglycemia and diabetes is required when being treated with ELIGARD [see Warnings and Precautions (5.3)].
Cardiovascular Disease
- Inform patients that there is an increased risk of myocardial infarction, sudden cardiac death, and stroke with ELIGARD treatment. Advise patients to immediately report signs and symptoms associated with these events to their healthcare provider for evaluation [see Warnings and Precautions (5.4)].
Injection Site Reactions
- Inform patients that injection site related adverse reactions may occur such as transient burning/stinging, pain, bruising, and redness. Advise patients to contact their healthcare provider if they experience rash or severe injection site reactions [see Adverse Reactions (6.1)].
Urogenital Disorders
- Advise patients that ELIGARD may cause impotence.
Infertility
- Inform patients that ELIGARD may cause infertility [see Use In Specific Populations (8.3)].
Rx only
Revised 04/23
Manufactured by: Tolmar, Fort Collins, CO 80526
©2022 Tolmar
04006171 Rev. 2 04/23
INSTRUCTIONS FOR USE
Follow the detailed instructions below to ensure correct preparation of ELIGARD prior to administration:
|
Step 1 Use aseptic technique throughout the procedure. Gloves are recommended during mixing and administration. Allow the product to reach room temperature before mixing. Once mixed, the product must be administered within 30 minutes or it should be discarded. On a clean field open the tray by tearing off the foil from the corner and remove the contents. Discard the desiccant pack. Remove the pre-connected syringe system from the tray. Open the sterile safety needle package by peeling back the paper tab. Note: Syringe A and Syringe B should not be lined-up yet. The product should only be administered with the co-packaged, sterile safety needle. |
|
 |
|
| Step 2
Grasp the latching button on the coupling device with your finger and thumb and press until you hear a snapping sound. The two syringes will be aligned. | 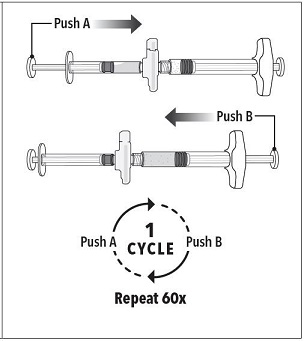 |
|
Step 3 Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprolide acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by pushing the contents back and forth between both syringes to obtain a uniform suspension.
Note: Product must be mixed as described; shaking will NOT provide adequate mixing. Do not bend. | 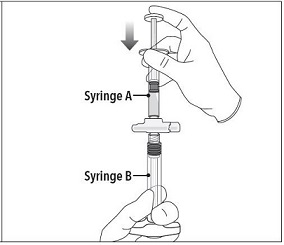 |
|
Step 4 After mixing, hold the syringes vertically (upright) with Syringe B (wide syringe) on the bottom. The syringes should remain securely coupled. Transfer all of the mixed product into Syringe B by depressing the Syringe A plunger and slightly withdrawing the Syringe B plunger. | 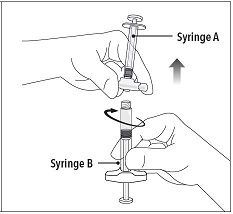 |
|
Step 5 While ensuring the Syringe A plunger is fully pushed down, hold the coupling device and unscrew Syringe B. This will disconnect Syringe B from the coupling device. Syringe A will remain attached to the coupling device.
Note: Small air bubbles will remain in the formulation – this is acceptable.
Do not purge the air bubbles from Syringe B as product may be lost! | 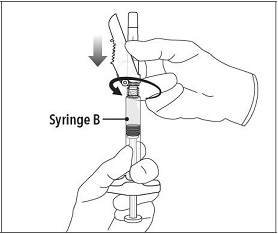 |
|
Step 6 Continue to hold Syringe B upright with the open end at the top. Hold back the white plunger on Syringe B to prevent loss of the product and attach the safety needle and cap. Gently screw clockwise with approximately a three-quarter turn until the safety needle and cap are secure.
Do not overtighten, as the needle hub may become damaged which could result in leakage of the product during injection. The safety shield may also be damaged if the safety needle and cap are screwed with too much force. | 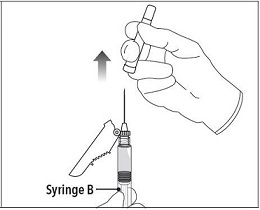 |
|
Step 7 Move the safety shield away from the needle and towards the syringe. Pull off the cap immediately prior to administration. | 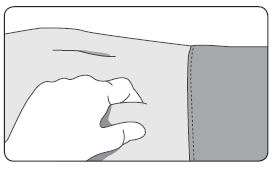 |
Note: Should the needle hub appear to be damaged, or leak, the product should NOT be used. The damaged safety needle and cap should NOT be replaced and the product should NOT be injected. In the event of damage to the needle hub, use a new replacement ELIGARD carton.
Follow the detailed instructions below to ensure correct administration of ELIGARD:
|
1. Select an injection site on the abdomen, upper buttocks, or another location with adequate amounts of subcutaneous tissue that does not have excessive pigment, nodules, lesions, or hair and hasn’t recently been used. 2. Cleanse the injection-site area with an alcohol swab (not enclosed). 3. Using the thumb and forefinger, grab and bunch the area of skin around the injection site. | 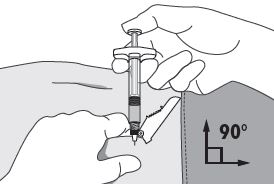 |
| 4. Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and fullness of the subcutaneous tissue and the length of the needle. After the needle is inserted, release the skin. 5. Inject the drug using a slow, steady push and press down on the plunger until the syringe is empty. Make sure all the drug has been injected before removing the needle. 6. Withdraw the needle quickly at the same 90° angle used for insertion. | 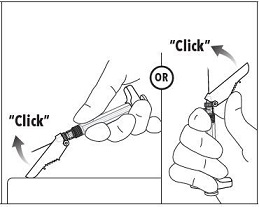 |
|
7. Immediately following the withdrawal of the needle, activate the safety shield using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place. 8. An audible and tactile “click” verifies a locked position. 9. Check to confirm the safety shield is fully engaged. Discard all components safely in an appropriate biohazard container. |  |
©2022 Tolmar
Manufactured by: Tolmar, Fort Collins, CO 80526 04006171 Rev. 2 04/23
| ELIGARD
leuprolide acetate injection, suspension, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TOLMAR Inc. (791156578) |