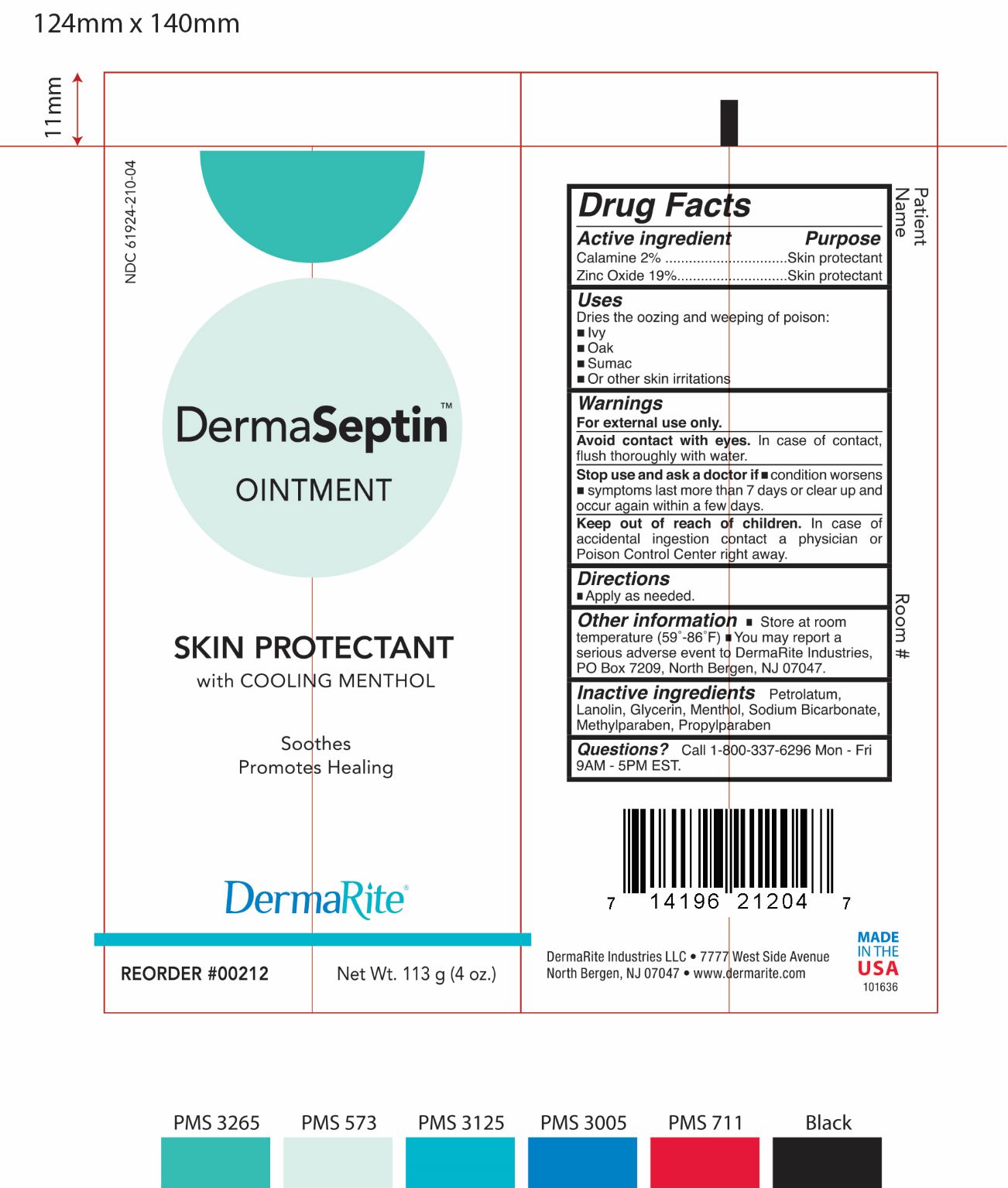
DERMASEPTIN- otc skin protectant drug products ointment
DermaRite Industries, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
- Zinc Oxide 19%
- Calamine 2%
Purpose:
- Zinc Oxide: Skin protectant
- Calamine : Skin protectant
Uses:
Dries the oozing and weeping of poison:
- Ivy
- Oak
- Sumac
- Or othe skin irritations
Warnings
-
For external use only.
-
Avoid contact with eyes. In case of contact, flush thoroughly with water.
-
Stop use and ask a doctor if condition worsens
- Symptoms last more than 7 days or clear up and occur again within a few days.
Warnings:
-
Keep out of reach of children. In case accidental ingestion contact a physician or Poison Control Center right away.
Directions
Applied as needed.
Other Information:
Store at room temperature (59°-86°F).
You may report a serious adverse event to DermaRite Industires, PO Box 7209, North Bergen, NJ 07047.
Inactive Ingredients:
Petrolatum, Lanolin, Glycerin, Menthol, Sodium Bicarbonate, methylparaben, Propylparaben
Questions
Call 1-800-337-6296 Mon-Fri 9AM-5PM EST.
Dermaseptin Package Label Principal Display Panel