WARNINGS SECTION
WARNINGS
For vaginal use only
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor
Ask a doctor before use if you have
• vaginal itching and discomfort for the first time
• lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge
• vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
• been exposed to the human immunodeficiency virus (HIV) that causes AIDS
Ask a doctor or pharmacist before use if you are taking the prescription blood thinning medicine warfarin, because bleeding or bruising may occur.
When using this product
• do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged by this product and fail to prevent pregnancy or sexually transmitted diseases (STDs).
• do not have vaginal intercourse
Stop use and ask a doctor if
• symptoms do not get better in 3 days
• symptoms last more than 7 days
• you get a rash, abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge
• In case of accidental ingestion, seek professional assistance or contact a poison control center immediately.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding, ask a health professional before use
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
• before using this product, read the enclosed brochure for complete instructions
• adults and children 12 years of age and over:
• insert 1 suppository into the vagina at bedtime for 7 nights in a row
• wash applicator after each use
• children under 12 years of age: ask a doctor
STORAGE AND HANDLING SECTION
• store at 15°-30°C (59°-86°F). Avoid heat over 30°C (86°F).
• do not use if suppository wrapper is missing or damaged (each suppository is individually wrapped)
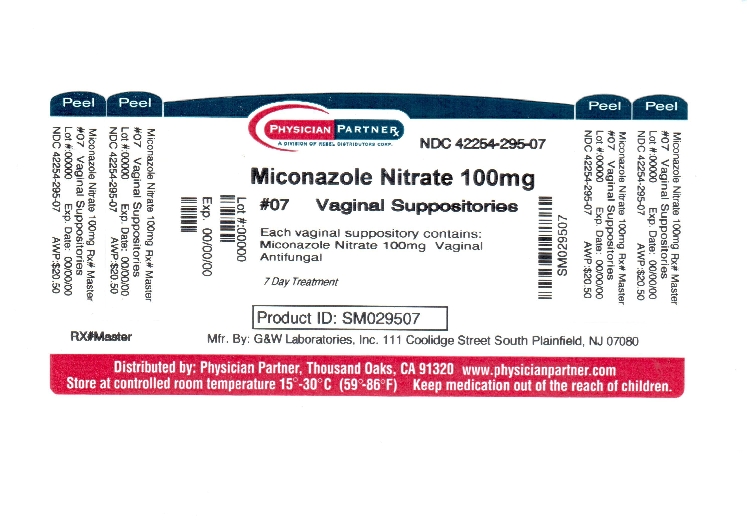
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Miconazole Nitrate Vaginal Suppositories USP, 100 mg
NDC 42254-295-07
7 Day Treatment
Miconazole Nitrate Vaginal Suppositories USP, 100 mg
Cures Most Vaginal Yeast Infections
Contains 7 Vaginal Suppositories
Drug Facts
Active ingredient (in each vaginal suppository) Purpose
Miconazole nitrate 100 mg.........................................................................................Vaginal antifungal
Use
• treats vaginal yeast infections
Warnings
For vaginal use only
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor
Ask a doctor before use if you have
• vaginal itching and discomfort for the first time
• lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge
• vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
• been exposed to the human immunodeficiency virus (HIV) that causes AIDS
Ask a doctor or pharmacist before use if you are taking the prescription blood thinning medicine warfarin, because bleeding or bruising may occur.
When using this product
• do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged by this product and fail to prevent pregnancy or sexually transmitted diseases (STDs).
• do not have vaginal intercourse
Stop use and ask a doctor if
• symptoms do not get better in 3 days
• symptoms last more than 7 days
• you get a rash, abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• before using this product, read the enclosed brochure for complete instructions
• adults and children 12 years of age and over:
• insert 1 suppository into the vagina at bedtime for 7 nights in a row • wash applicator after each use
• children under 12 years of age: ask a doctor
Drug Facts (continued)
Other information
• store at 15°-30°C (59°-86°F). Avoid heat over 30°C (86°F).
• do not use if suppository wrapper is missing or damaged (each suppository is individually wrapped)
Inactive ingredienthydrogenated vegetable oil base
Drug Facts (continued)
Questions? call 1-800-922-1038
Manufactured by: G&W Laboratories, Inc.
111 Coolidge Street
South Plainfield, NJ 07080
Visit our website @ www.gwlabs.com
Repackaged by: Rebel Distributors Corp.
3607 Old Conejo Rd.
Thousand Oaks, CA 91320
TAMPER-EVIDENT: For your safety each suppository is individually wrapped. If a suppository is unwrapped or there are signs of tampering, DO NOT USE.