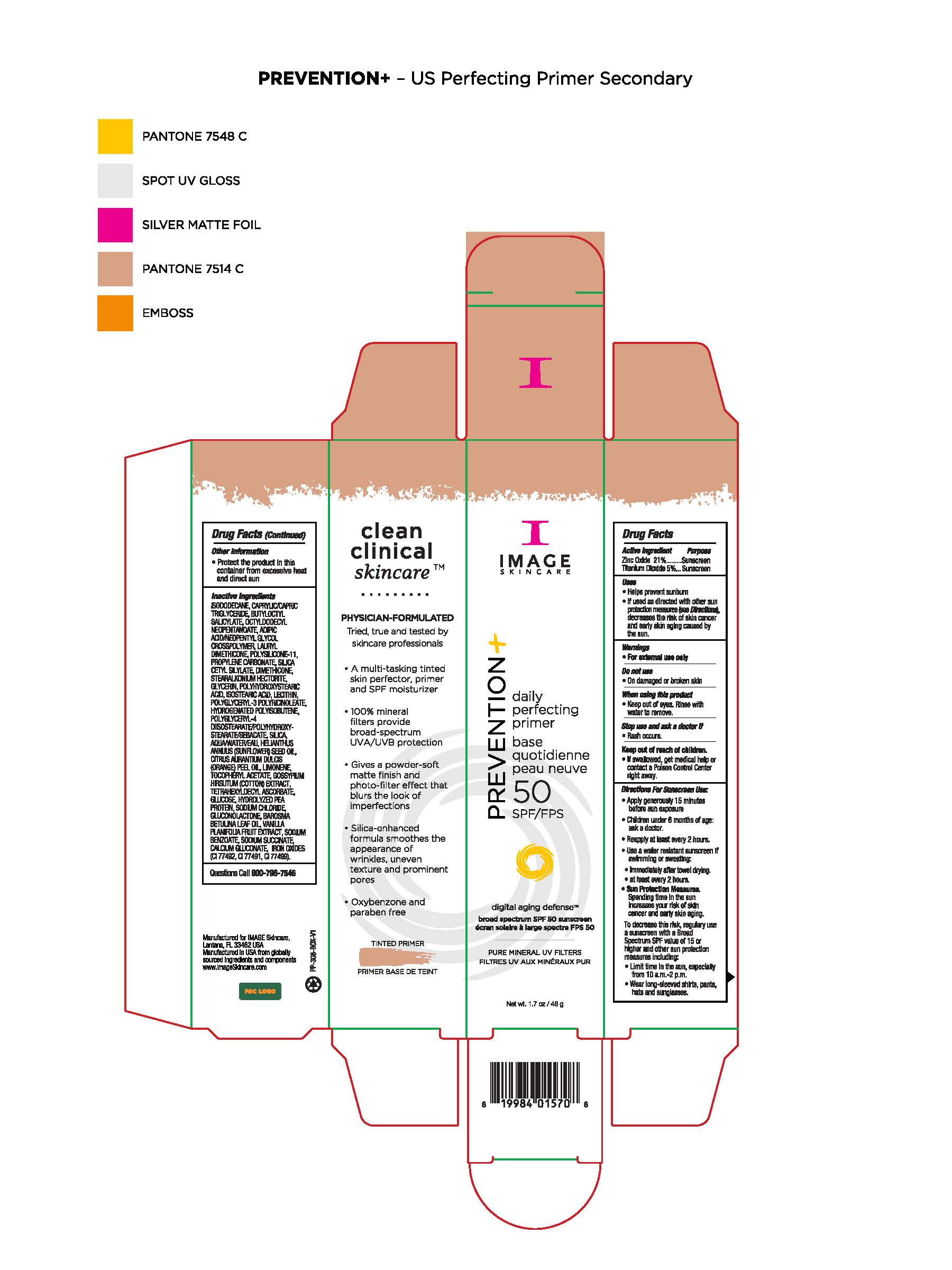
Warnings
For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs. If swallowed, get medical help or contact a Poison Control Center right away.
Uses
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Uses
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
Apply generously 15 minutes before sun exposure. Children under 6 months of age: ask a doctor. Reapply at least every 2 hours. Use a water resistant sunscreen if swimming or sweating. Immediately after towel drying. At least every 2 hours. Use a water resistant sunscreen when swimming or sweating, immediately after towel drying, at least every two hours. Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am to 2pm, wear long-sleeved shirts, pants, hats and sunglasses.
Inactive Ingredients
idododecane, caprylic/capric triglyceride, butyloctyl salicylate, octyldodecyl neopentanoate, adipic acid/neopentyl glycol crosspolymer, lauryl dimethicone, polysilicone-11, propylene carbonate, silica cetyl silylate, dimethicone, stearalkonium hectorite, glycerin, polyhydroxystearic acid, isostearic acid, lecithin, polyglyceryl-3 polyricinoleate, hydrogenated polyisobutene, polyglyceryl-4 diisotearate/polyhydroxystearate/sebacate, silica, water, helinathus annuus (sunflower) seed oil, citrus aurantium dulcis (orange) peel oil, limonene, tocopheryl acetate, gossypium hirsuthum (cotton) extract, tetrahexyldecyl ascorbate, glucose, hydrolyzed pea protein, sodium chloride, glyconolactone, barosma betulina leaf oil, vanilla planifolia fruit extract, sodium benzoate, sodium succinate, calcium glyconate, iron oxides (CI77492, CI77491, CI77499)