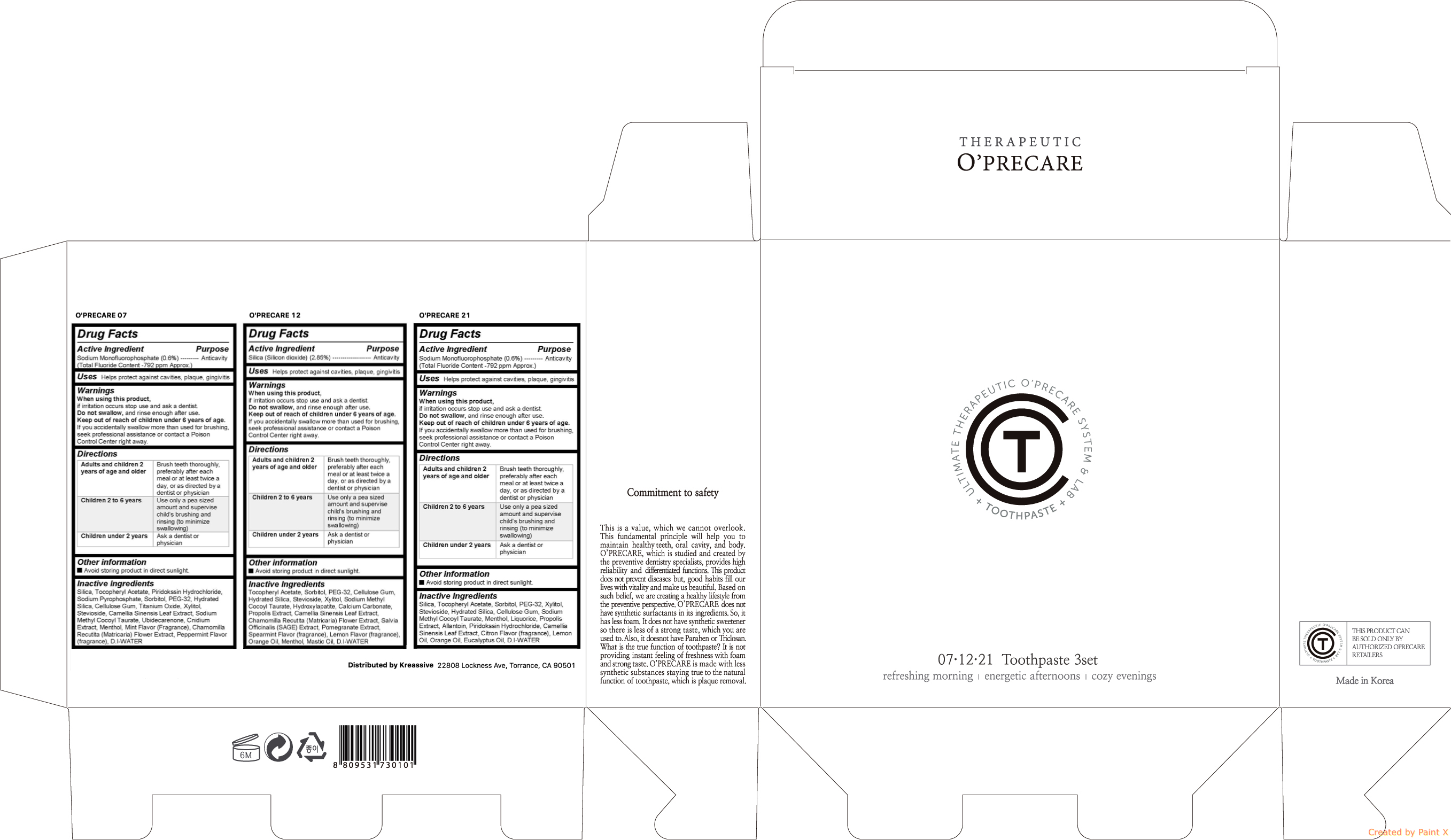
Active ingredient
| O'PRECARE 07 | Sodium Monofluorophosphate (0.6%) |
| O'PRECARE 12 | Silica (Silicon dioxide) (2.85%) |
| O'PRECARE 21 | Sodium Monofluorophosphate (0.6%) |
Warnings
When using this product, if irritation occurs stop use and ask a dentist. Do not swallow, and rinse enough after use. Keep out of reach of children under 6 years of age. If you accidentally swallow more than used for brushing, seek professional assistance or contact a Poison Control Center right away.
Directions
| Adults and children 2 years of age and older | Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| Children 2 to 6 years | Use only a pea sized amount and supervise childs brushing and rinsing (to minimize swallowing) |
| Children under 2 years | Ask a dentist or physician |
Keep out of reach of children
Keep out of reach of children under 6 years of age. If you accidentally swallow more than used for brushing, seek professional assistance or contact a Poison Control Center right away.
Inactive ingredients
| O'PRECARE 07 | Silica, Tocopheryl Acetate, Piridokssin Hydrochloride, Sodium Pyrophosphate, Sorbitol, PEG-32, Hydrated Silica, Cellulose Gum, Titanium Oxide, Xylitol, Stevioside, Camellia Sinensis Leaf Extract, Sodium Methyl Cocoyl Taurate, Ubidecarenone, Cnidium Extract, Menthol, Mint Flavor (Fragrance), Chamomilla Recutita (Matricaria) Flower Extract, Peppermint Flavor (fragrance), D.I-WATER |
| O'PRECARE 12 | Tocopheryl Acetate, Sorbitol, PEG-32, Cellulose Gum, Hydrated Silica, Stevioside, Xylitol, Sodium Methyl Cocoyl Taurate, Hydroxylapatite, Calcium Carbonate, Propolis Extract, Camellia Sinensis Leaf Extract, Chamomilla Recutita (Matricaria) Flower Extract, Salvia Officinalis (SAGE) Extract, Pomegranate Extract, Spearmint Flavor (fragrance), Lemon Flavor (fragrance), Orange Oil, Menthol, Mastic Oil, D.I-WATER |
| O'PRECARE 21 | Silica, Tocopheryl Acetate, Piridokssin Hydrochloride, Sodium Pyrophosphate, Sorbitol, PEG-32, Hydrated Silica, Cellulose Gum, Titanium Oxide, Xylitol, Stevioside, Camellia Sinensis Leaf Extract, Sodium Methyl Cocoyl Taurate, Ubidecarenone, Cnidium Extract, Menthol, Mint Flavor (Fragrance), Chamomilla Recutita (Matricaria) Flower Extract, Peppermint Flavor (fragrance), D.I-WATER |