FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Complicated Skin and Skin Structure Infections (Adult Patients and Pediatric Patients 3 Months of age and older only)
Meropenem for injection is indicated for the treatment of complicated skin and skin structure infections (cSSSI) due to Staphylococcus aureus(methicillin-susceptible isolates only), Streptococcus pyogenes, Streptococcus agalactiae, viridans group streptococci, Enterococcus faecalis(vancomycin-susceptible isolates only), Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Bacteroides fragilis, and Peptostreptococcusspecies .
1.2 Complicated Intra-abdominal Infections (Adult and Pediatric Patients)
Meropenem for injection is indicated for the treatment of complicated appendicitis and peritonitis caused by viridans group streptococci, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacteroides fragilis, B. thetaiotaomicron, and Peptostreptococcusspecies .
1.3 Bacterial Meningitis (Pediatric Patients 3 Months of age and older only)
Meropenem for injection is indicated for the treatment of bacterial meningitis caused by Haemophilus influenzae, and Neisseria meningitidis and penicillin-susceptible isolates of Streptococcus pneumoniae.
Meropenem for injection has been found to be effective in eliminating concurrent bacteremia in association with bacterial meningitis.
1.4 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of meropenem for injection and other antibacterial drugs, meropenem for injection should only be used to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients
The recommended dose of meropenem for injection is 500 mg given every 8 hours for skin and skin structure infections and 1 gram given every 8 hours for intra-abdominal infections. When treating complicated skin and skin structure infections caused by P.aeruginosa, a dose of 1 gram every 8 hours is recommended.
Meropenem for injection should be administered by intravenous infusion over approximately 15 minutes to 30 minutes. Doses of 1 gram may also be administered as an intravenous bolus injection (5 mL to 20 mL) over approximately 3 minutes to 5 minutes.
2.2 Use in Adult Patients with Renal Impairment
Dosage should be reduced in patients with creatinine clearance of 50 mL/min or less. (See dosing Table 1 below.)
When only serum creatinine is available, the following formula (Cockcroft and Gault equation) 1may be used to estimate creatinine clearance.
Males: Creatinine Clearance (mL/min) =
| Weight (kg) × (140 - age) |
| 72 × serum creatinine (mg/dL) |
Females: 0.85 × above value
Table 1: Recommended Meropenem for Injection Dosage Schedule for Adult Patients with Renal Impairment
| Creatinine Clearance
(mL/min) | Dose (dependent on type of infection) | Dosing Interval |
|---|---|---|
| Greater than 50 | Recommended dose (500 mg cSSSI and 1 gram Intra-abdominal) | Every 8 hours |
| 26-50 | Recommended dose | Every 12 hours |
| 10-25 | One-half recommended dose | Every 12 hours |
| Less than 10 | One-half recommended dose | Every 24 hours |
There is inadequate information regarding the use of meropenem for injection in patients on hemodialysis or peritoneal dialysis.
2.3 Use in Pediatric Patients
Pediatric Patients 3 Months of Age and Older
- For pediatric patients 3 months of age and older, the Meropenem for Injection dose is 10 mg/kg, 20 mg/kg or 40 mg/kg every 8 hours (maximum dose is 2 grams every 8 hours), depending on the type of infection (cSSSI, cIAI, intra-abdominal infection or meningitis). See dosing Table 2 below.
- For pediatric patients weighing over 50 kg administer meropenem for injection at a dose of 500 mg every 8 hours for cSSSI, 1 gram every 8 hours for cIAI and 2 grams every 8 hours for meningitis.
- Administer meropenem for injection as an intravenous infusion over approximately 15 minutes to 30 minutes or as an intravenous bolus injection (5 mL to 20 mL) over approximately 3 minutes to 5 minutes.
- There is limited safety data available to support the administration of a 40 mg/kg (up to a maximum of 2 grams) bolus dose.
Table 2: Recommended Meropenem for Injection Dosage Schedule for Pediatric Patients 3 Months of Age and Older with Normal Renal Function
| Type of Infection | Dose (mg/kg) | Up to a Maximum Dose | Dosing Interval |
|---|---|---|---|
| Complicated skin and skin structure Infections | 10 | 500 mg | Every 8 hours |
| Complicated Intra-abdominal Infections | 20 | 1 gram | Every 8 hours |
| Meningitis | 40 | 2 grams | Every 8 hours |
There is no experience in pediatric patients with renal impairment.
When treating cSSSI caused by P. aeruginosa, a dose of 20 mg/kg (or 1 gram for pediatric patients weighing over 50 kg) every 8 hours is recommended.
Pediatric Patients Less Than 3 Months of Age
For pediatric patients (with normal renal function) less than 3 months of age, with complicated intra-abdominal infections, the meropenem for injection dose is based on gestational age (GA) and postnatal age (PNA). See dosing Table 3 below.
Meropenem for injection should be given as intravenous infusion over 30 minutes.
Table 3: Recommended Meropenem for Injection Dosage Schedule for Pediatric Patients Less than 3 Months of Age with Complicated Intra-abdominal Infections and Normal Renal Function
| Age Group | Dose (mg/kg) | Dose Interval |
|---|---|---|
| Infants less than 32 weeks GA and PNA less than 2 weeks | 20 | Every 12 hours |
| Infants less than 32 weeks GA and PNA 2 weeks and older | 20 | Every 8 hours |
| Infants 32 weeks and older GA and PNA less than 2 weeks | 20 | Every 8 hours |
| Infants 32 weeks and older GA and PNA 2 weeks and older | 30 | Every 8 hours |
There is no experience in pediatric patients with renal impairment.
2.4 Preparation and Administration of Meropenem for Injection
Important Administration Instructions:
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
For Intravenous Bolus Administration
Re-constitute injection vials (500 mg and 1 gram) with sterile Water for Injection (see Table 4 below). Shake to dissolve and let stand until clear.
Table 4: Volume of Sterile Water for Injection for Reconstitution of Injection Vials
| Vial Size | Amount of Diluent Added (mL) | Approximate Withdrawable Volume (mL) | Approximate Average Concentration (mg/mL) |
|---|---|---|---|
| 500 mg | 10 | 10 | 50 |
| 1 gram | 20 | 20 | 50 |
For Infusion
- Injection vials (500 mg and 1 gram) may be directly re-constituted with a compatible infusion fluid.
- Alternatively, an injection vial may be re-constituted, then the resulting solution added to an intravenous container and further diluted with an appropriate infusion fluid [see Dosage and Administration (2.5)and (2.6)].
- Do not use flexible container in series connections.
2.5 Compatibility
Compatibility of meropenem for injection with other drugs has not been established. Meropenem for injection should not be mixed with or physically added to solutions containing other drugs.
2.6 Stability and Storage
Freshly prepared solutions of meropenem for injection should be used. However, re-constituted solutions of meropenem for injection maintain satisfactory potency under the conditions described below. Solutions of intravenous meropenem for injection should not be frozen.
Intravenous Bolus Administration
Meropenem for injection vials re-constituted with sterile Water for Injection for bolus administration (up to 50 mg/mL of meropenem for injection) may be stored for up to 3 hours at up to 25°C (77°F) or for 13 hours at up to 5°C (41°F).
Intravenous Infusion Administration
Solutions prepared for infusion (meropenem for injection concentrations ranging from 1 mg/mL to 20 mg/mL) re-constituted with Sodium Chloride Injection 0.9% may be stored for 1 hour at up to 25°C (77°F) or 15 hours at up to 5°C (41°F).
Solutions prepared for infusion (meropenem for injection concentrations ranging from 1 mg/mL to 20 mg/mL) re-constituted with Dextrose Injection 5% should be used immediately.
3 DOSAGE FORMS AND STRENGTHS
Single dose clear glass vials of meropenem for injection containing 500 mg or 1 gram (as the trihydrate blended with anhydrous sodium carbonate for re-constitution) of sterile meropenem powder.
4 CONTRAINDICATIONS
Meropenem is contraindicated in patients with known hypersensitivity to any component of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta (β)-lactams.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with β-lactams. These reactions are more likely to occur in individuals with a history of sensitivity to multiple allergens.
There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe hypersensitivity reactions when treated with another β-lactam. Before initiating therapy with meropenem, it is important to inquire about previous hypersensitivity reactions to penicillins, cephalosporins, other β-lactams, and other allergens. If an allergic reaction to meropenem occurs, discontinue the drug immediately.
5.2 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), erythema multiforme (EM) and acute generalized exanthematous pustulosis (AGEP) have been reported in patients receiving meropenem [see Adverse Reactions (6.2) ]. If signs and symptoms suggestive of these reactions appear, meropenem should be withdrawn immediately and an alternative treatment should be considered.
5.3 Seizure Potential
Seizures and other adverse CNS experiences have been reported during treatment with meropenem. These experiences have occurred most commonly in patients with CNS disorders (e.g., brain lesions or history of seizures) or with bacterial meningitis and/or compromised renal function [see Adverse Reactions (6.1), Drug Interactions (7.2)].
During clinical investigations, 2904 immunocompetent adult patients were treated for non-CNS infections with the overall seizure rate being 0.7% (based on 20 patients with this adverse event). All meropenem-treated patients with seizures had pre-existing contributing factors. Among these are included prior history of seizures or CNS abnormality and concomitant medications with seizure potential. Dosage adjustment is recommended in patients with advanced age and/or adult patients with creatinine clearance of 50 mL/min or less [see Dosage and Administration (2.2)].
Close adherence to the recommended dosage regimens is urged, especially in patients with known factors that predispose to convulsive activity. Continue anti-convulsant therapy in patients with known seizure disorders. If focal tremors, myoclonus, or seizures occur, evaluate neurologically, placed on anti-convulsant therapy if not already instituted, and re-examine the dosage of meropenem to determine whether it should be decreased or discontinued.
5.4 Risk of Breakthrough Seizures Due to Drug Interaction with Valproic Acid
The concomitant use of meropenem and valproic acid or divalproex sodium is generally not recommended. Case reports in the literature have shown that co-administration of carbapenems, including meropenem, to patients receiving valproic acid or divalproex sodium results in a reduction in valproic acid concentrations. The valproic acid concentrations may drop below the therapeutic range as a result of this interaction, therefore increasing the risk of breakthrough seizures. Increasing the dose of valproic acid or divalproex sodium may not be sufficient to overcome this interaction. Consider administration of antibacterial drugs other than carbapenems to treat infections in patients whose seizures are well controlled on valproic acid or divalproex sodium. If administration of meropenem is necessary, consider supplemental anti-convulsant therapy [see Drug Interactions (7.2)].
5.5 Clostridium difficile-associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including meropenem, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficileproduces toxins A and B which contribute to the development of CDAD. Hypertoxin producing isolates of C. difficilecause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficilemay need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.6 Development of Drug-Resistant Bacteria
Prescribing meropenem in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.7 Overgrowth of Nonsusceptible Organisms
As with other broad-spectrum antibacterial drugs, prolonged use of meropenem may result in overgrowth of nonsusceptible organisms. Repeated evaluation of the patient is essential. If superinfection does occur during therapy, appropriate measures should be taken.
5.8 Thrombocytopenia
In patients with renal impairment, thrombocytopenia has been observed but no clinical bleeding reported [see Dosage and Administration (2.2), Adverse Reactions (6.1), Use In Specific Populations (8.5), (8.6), Clinical Pharmacology (12.3)].
5.9 Potential for Neuromotor Impairment
Alert patients receiving meropenem on an outpatient basis regarding adverse events such as seizures, delirium, headaches and/or paresthesias that could interfere with mental alertness and/or cause motor impairment. Until it is reasonably well established that meropenem is well tolerated, advise patients not to operate machinery or motorized vehicles [see Adverse Reactions (6.1)].
6 ADVERSE REACTIONS
The following are discussed in greater detail in other sections of labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.2)]
- Seizure Potential [see Warnings and Precautions (5.3)]
- Risk of Breakthrough Seizures Due to Drug Interaction with Valproic Acid [see Warnings and Precautions (5.4)]
- Clostridium difficile- associated Diarrhea [see Warnings and Precautions (5.5)]
- Development of Drug-Resistant Bacteria [see Warnings and Precautions (5.6)]
- Overgrowth of Nonsusceptible Organisms [see Warnings and Precautions (5.7)]
- Thrombocytopenia [see Warnings and Precautions (5.8)]
- Potential for Neuromotor Impairment [see Warnings and Precautions (5.9)]
6.1 Adverse Reactions from Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Patients:
During clinical investigations, 2904 immunocompetent adult patients were treated for non-CNS infections with meropenem (500 mg or 1 gram every 8 hours). Deaths in 5 patients were assessed as possibly related to meropenem; 36 (1.2%) patients had meropenem discontinued because of adverse events. Many patients in these trials were severely ill and had multiple background diseases, physiological impairments and were receiving multiple other drug therapies. In the seriously ill patient population, it was not possible to determine the relationship between observed adverse events and therapy with meropenem.
The following adverse reaction frequencies were derived from the clinical trials in the 2904 patients treated with meropenem.
Local Adverse Reactions
Local adverse events that were reported with meropenem were as follows:
| Inflammation at the injection site | 2.4% | |
| Injection site reaction | 0.9% | |
| Phlebitis/thrombophlebitis | 0.8% | |
| Pain at the injection site | 0.4% | |
| Edema at the injection site | 0.2% |
Systemic Adverse Reactions
Systemic adverse events that were reported with meropenem occurring in greater than 1.0% of the patients were diarrhea (4.8%), nausea/vomiting (3.6%), headache (2.3%), rash (1.9%), sepsis (1.6%), constipation (1.4%), apnea (1.3%), shock (1.2%), and pruritus (1.2%).
Additional systemic adverse events that were reported with meropenem and occurring in less than or equal to 1.0% but greater than 0.1% of the patients are listed below within each body system in order of decreasing frequency:
Bleeding events were seen as follows: gastrointestinal hemorrhage (0.5%), melena (0.3%), epistaxis (0.2%), hemoperitoneum (0.2%).
Body as a Whole: pain, abdominal pain, chest pain, fever, back pain, abdominal enlargement, chills, pelvic pain
Cardiovascular: heart failure, heart arrest, tachycardia, hypertension, myocardial infarction, pulmonary embolus, bradycardia, hypotension, syncope
Digestive System: oral moniliasis, anorexia, cholestatic jaundice/jaundice, flatulence, ileus, hepatic failure, dyspepsia, intestinal obstruction
Hemic/Lymphatic: anemia, hypochromic anemia, hypervolemia
Metabolic/Nutritional: peripheral edema, hypoxia
Nervous System: insomnia, agitation, delirium, confusion, dizziness, seizure, nervousness, paresthesia, hallucinations, somnolence, anxiety, depression, asthenia [see Warnings and Precautions (5.3)and (5.9)]
Respiratory: respiratory disorder, dyspnea, pleural effusion, asthma, cough increased, lung edema
Skin and Appendages: urticaria, sweating, skin ulcer
Urogenital System: dysuria, kidney failure, vaginal moniliasis, urinary incontinence
Adverse Laboratory Changes
Adverse laboratory changes that were reported and occurring in greater than 0.2% of the patients were as follows:
Hepatic:increased alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase, lactose dehydrogenase (LDH), and bilirubin
Hematologic:increased platelets, increased eosinophils, decreased platelets, decreased hemoglobin, decreased hematocrit, decreased white blood cell (WBC), shortened prothrombin time and shortened partial thromboplastin time, leukocytosis, hypokalemia
Renal:increased creatinine and increased blood urea nitrogen (BUN)
Urinalysis:presence of red blood cells
Complicated Skin and Skin Structure Infections
In a study of complicated skin and skin structure infections, the adverse reactions were similar to those listed above. The most common adverse events occurring in greater than 5% of the patients were: headache (7.8%), nausea (7.8%), constipation (7.0%), diarrhea (7.0%), anemia (5.5%), and pain (5.1%). Adverse events with an incidence of greater than 1%, and not listed above, include: pharyngitis, accidental injury, gastrointestinal disorder, hypoglycemia, peripheral vascular disorder, and pneumonia.
Patients with Renal Impairment:
For patients with varying degrees of renal impairment, the incidence of heart failure, kidney failure, seizure and shock reported with meropenem, increased in patients with moderately severe renal impairment (creatinine clearance 10 to 26 mL/min) [see Dosage and Administration (2.2), Warnings and Precautions (5.9), Use in Specific Populations (8.5), (8.6), Clinical Pharmacology (12.3)].
Pediatric Patients:
Systemic and Local Adverse Reactions
Pediatric Patients with Serious Bacterial Infections (excluding Bacterial Meningitis):
Meropenem was studied in 515 pediatric patients (3 months to less than 13 years of age) with serious bacterial infections (excluding meningitis, see next section) at dosages of 10 mg/kg to 20 mg/kg every 8 hours. The types of systemic and local adverse events seen in these patients are similar to the adults, with the most common adverse events reported as possibly, probably, or definitely related to meropenem and their rates of occurrence as follows:
| Diarrhea | 3.5% | |
| Rash | 1.6% | |
| Nausea and Vomiting | 0.8% |
Pediatric Patients with Bacterial Meningitis:
Meropenem was studied in 321 pediatric patients (3 months to less than 17 years of age) with meningitis at a dosage of 40 mg/kg every 8 hours. The types of systemic and local adverse events seen in these patients are similar to the adults, with the most common adverse reactions reported as possibly, probably, or definitely related to meropenem and their rates of occurrence as follows:
| Diarrhea | 4.7% | |
| Rash (mostly diaper area moniliasis) | 3.1% | |
| Oral Moniliasis | 1.9% | |
| Glossitis | 1.0% |
In the meningitis studies, the rates of seizure activity during therapy were comparable between patients with no CNS abnormalities who received meropenem and those who received comparator agents (either cefotaxime or ceftriaxone). In the meropenem treated group, 12/15 patients with seizures had late onset seizures (defined as occurring on day 3 or later) versus 7/20 in the comparator arm. The meropenem group had a statistically higher number of patients with transient elevation of liver enzymes.
Pediatric Patients (Neonates and Infants less than 3 months of Age):
Meropenem was studied in 200 neonates and infants less than 3 months of age. The study was open-label, uncontrolled, 98% of the infants received concomitant medications, and the majority of adverse events were reported in neonates less than 32 weeks gestational age and critically ill at baseline, making it difficult to assess the relationship of the adverse events to meropenem.
The adverse reactions seen in these patients that were reported and their rates of occurrence are as follows:
| Convulsion | 5.0% | |
| Hyperbilirubinemia (conjugated) | 4.5% | |
| Vomiting | 2.5% |
Adverse Laboratory Changes in Pediatric Patients:
Laboratory changes seen in the pediatric studies, including the meningitis studies, were similar to those reported in the adult studies.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of meropenem. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Worldwide post-marketing adverse reactions not otherwise listed in the Adverse Reactions from Clinical Trials section of this prescribing information and reported as possibly, probably, or definitely drug related are listed within each body system in order of decreasing severity.
Blood and Lymphatic System Disorders: agranulocytosis, neutropenia, and leukopenia; a positive direct or indirect Coombs test, and hemolytic anemia.
Immune System Disorders:angioedema.
Skin and Subcutaneous Disorders:Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), erythema multiforme and acute generalized exanthematous pustulosis.
7 DRUG INTERACTIONS
7.1 Probenecid
Probenecid competes with meropenem for active tubular secretion, resulting in increased plasma concentrations of meropenem. Co-administration of probenecid with meropenem is not recommended.
7.2 Valproic Acid
Case reports in the literature have shown that co-administration of carbapenems, including meropenem, to patients receiving valproic acid or divalproex sodium results in a reduction in valproic acid concentrations. The valproic acid concentrations may drop below the therapeutic range as a result of this interaction, therefore increasing the risk of breakthrough seizures. Although the mechanism of this interaction is unknown, data from in vitroand animal studies suggest that carbapenems may inhibit the hydrolysis of valproic acid's glucuronide metabolite (VPA-g) back to valproic acid, thus decreasing the serum concentrations of valproic acid. If administration of meropenem is necessary, then supplemental anti-convulsant therapy should be considered [see Warnings and Precautions (5.4)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient human data to establish whether there is a drug-associated risk of major birth defects or miscarriages with meropenem in pregnant women.
No fetal toxicity or malformations were observed in pregnant rats and Cynomolgus monkeys administered intravenous meropenem during organogenesis at doses up to 2.4 and 2.3 times the maximum recommended human dose (MRHD) based on body surface area comparison, respectively. In rats administered intravenous meropenem in late pregnancy and during the lactation period, there were no adverse effects on offspring at doses equivalent to approximately 3.2 times the MRHD based on body surface area comparison (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Meropenem administered to pregnant rats during organogenesis (Gestation Day 6 to Gestation Day 17) in intravenous doses of 240, 500, and 750 mg/kg/day was associated with mild maternal weight loss at all doses, but did not produce malformations or fetal toxicity. The no-observed-adverse-effect-level (NOAEL) for fetal toxicity in this study was considered to be the high dose of 750 mg/kg/day (equivalent to approximately 2.4 times the MRHD of 1 gram every 8 hours based on body surface area comparison). Meropenem administered intravenously to pregnant Cynomolgus monkeys during organogenesis from Day 20 to 50 after mating at doses of 120, 240, and 360 mg/kg/day did not produce maternal or fetal toxicity at the NOAEL dose of 360 mg/kg/day (approximately 2.3 times the MRHD based on body surface area comparison).
In a peri-postnatal study in rats described in the published literature 2, intravenous meropenem was administered to dams from Gestation Day 17 until Lactation Day 21 at doses of 240, 500, and 1000 mg/kg/day. There were no adverse effects in the dams and no adverse effects in the first generation offspring (including developmental, behavioral, and functional assessments and reproductive parameters) except that female offspring exhibited lowered body weights which continued during gestation and nursing of the second generation offspring. Second generation offspring showed no meropenem-related effects. The NOAEL value was considered to be 1000 mg/kg/day (approximately 3.2 times the MRHD based on body surface area comparisons).
8.2 Lactation
Risk Summary
Meropenem has been reported to be excreted in human milk. No information is available on the effects of meropenem on the breast-fed child or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for meropenem and any potential adverse effects on the breast-fed child from meropenem or from the underlying maternal conditions.
8.4 Pediatric Use
The safety and effectiveness of meropenem have been established for pediatric patients 3 months of age and older with complicated skin and skin structure infections and bacterial meningitis, and for pediatric patients of all ages with complicated intra-abdominal infections.
Skin and Skin Structure Infections
Use of meropenem in pediatric patients 3 months of age and older with complicated skin and skin structure infections is supported by evidence from an adequate and well-controlled study in adults and additional data from pediatric pharmacokinetics studies [see Indications and Usage (1.3), Dosage and Administration (2.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.1)].
Intra-abdominal Infections
Use of meropenem in pediatric patients 3 months of age and older with intra-abdominal infections is supported by evidence from adequate and well-controlled studies in adults with additional data from pediatric pharmacokinetics studies and controlled clinical trials in pediatric patients. Use of meropenem in pediatric patients less than 3 months of age with intra-abdominal infections is supported by evidence from adequate and well-controlled studies in adults with additional data from a pediatric pharmacokinetic and safety study [see Indications and Usage (1.2), Dosage and Administration (2.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3)and Clinical Studies (14.2)].
Bacterial Meningitis
Use of meropenem in pediatric patients 3 months of age and older with bacterial meningitis is supported by evidence from adequate and well-controlled studies in the pediatric population [see Indications and Usage (1.3), Dosage and Administration (2.3), Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.3)].
8.5 Geriatric Use
Of the total number of subjects in clinical studies of meropenem, approximately 1100 (30%) were 65 years of age and older, while 400 (11%) were 75 years and older. Additionally, in a study of 511 patients with complicated skin and skin structure infections, 93 (18%) were 65 years of age and older, while 38 (7%) were 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects; spontaneous reports and other reported clinical experience have not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Meropenem is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with renal impairment. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
A pharmacokinetic study with meropenem in elderly patients has shown a reduction in the plasma clearance of meropenem that correlates with age-associated reduction in creatinine clearance [see Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
Dosage adjustment is necessary in patients with creatinine clearance 50 mL/min or less [see Dosage and Administration (2.2), Warnings and Precautions (5.8), Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In mice and rats, large intravenous doses of meropenem (2200 mg/kg to 4000 mg/kg) have been associated with ataxia, dyspnea, convulsions, and mortalities.
Intentional overdosing of meropenem is unlikely, although accidental overdosing might occur if large doses are given to patients with reduced renal function. The largest dose of meropenem administered in clinical trials has been 2 grams given intravenously every 8 hours. At this dosage, no adverse pharmacological effects or increased safety risks have been observed.
Limited postmarketing experience indicates that if adverse events occur following overdosage, they are consistent with the adverse event profile described in the Adverse Reactions section and are generally mild in severity and resolve on withdrawal or dose reduction. Consider symptomatic treatments. In individuals with normal renal function, rapid renal elimination takes place. Meropenem and its metabolite are readily dialyzable and effectively removed by hemodialysis; however, no information is available on the use of hemodialysis to treat overdosage.
11 DESCRIPTION
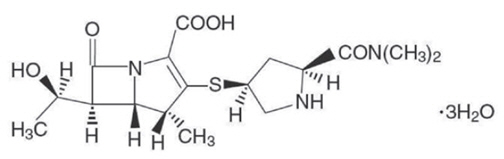
Meropenem for injection is a sterile, pyrogen-free, synthetic, carbapenem antibacterial for intravenous administration. It is (4R,5S,6S)-3- [[(3S,5S)-5-(Dimethylcarbamoyl)-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxilic acid trihydrate. Its empirical formula is C 17H 25N 3O 5S∙3H 2O with a molecular weight of 437.52. Its structural formula is:
Meropenem for injection is a white to pale yellow crystalline powder. The solution varies from colorless to yellow depending on the concentration. The pH of freshly constituted solutions is between 7.3 and 8.3.
Meropenem is soluble in 5% monobasic potassium phosphate solution, sparingly soluble in water, very slightly soluble in hydrated ethanol, and practically insoluble in acetone or ether.
When re-constituted as instructed, each 1 gram meropenem for injection vial will deliver 1 gram of meropenem and 90.2 mg of sodium as sodium carbonate (3.92 mEq). Each 500 mg meropenem for injection vial will deliver 500 mg meropenem and 45.1 mg of sodium as sodium carbonate (1.96 mEq) [see Dosage and Administration (2.4)].
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
The percentage of time of a dosing interval that unbound plasma concentration of meropenem exceeds the meropenem minimum inhibitory concentration (MIC) against the infecting organism has been shown to best correlate with efficacy in animal and in vitro models of infection.
12.3 Pharmacokinetics
Plasma Concentrations
At the end of a 30-minute intravenous infusion of a single dose of meropenem for injection in healthy volunteers, mean peak plasma concentrations of meropenem are approximately 23 mcg/mL (range 14 to 26) for the 500 mg dose and 49 mcg/mL (range 39-58) for the 1 gram dose. A 5-minute intravenous bolus injection of meropenem for injection in healthy volunteers results in mean peak plasma concentrations of approximately 45 mcg/mL (range 18 to 65) for the 500 mg dose and 112 mcg/mL (range 83 to 140) for the 1 gram dose.
Following intravenous doses of 500 mg, mean plasma concentrations of meropenem usually decline to approximately 1 mcg/mL at 6 hours after administration.
No accumulation of meropenem in plasma was observed with regimens using 500 mg administered every 8 hours or 1 gram administered every 6 hours in healthy volunteers with normal renal function.
Distribution
The plasma protein binding of meropenem is approximately 2%.
After a single intravenous dose of meropenem, the highest mean concentrations of meropenem were found in tissues and fluids at 1 hour (0.5 hours to 1.5 hours) after the start of infusion, except where indicated in the tissues and fluids listed in Table 5 below.
Table 5: Meropenem Concentrations in Selected Tissues (Highest Concentrations Reported)
| Tissue | Intravenous. Dose (gram) | Number of Samples | Mean
[mcg/mL or mcg/(gram)] * | Range
[mcg/mL or mcg/(gram)] |
|---|---|---|---|---|
| Endometrium | 0.5 | 7 | 4.2 | 1.7-10.2 |
| Myometrium | 0.5 | 15 | 3.8 | 0.4-8.1 |
| Ovary | 0.5 | 8 | 2.8 | 0.8-4.8 |
| Cervix | 0.5 | 2 | 7 | 5.4-8.5 |
| Fallopian tube | 0.5 | 9 | 1.7 | 0.3-3.4 |
| Skin | 0.5 | 22 | 3.3 | 0.5-12.6 |
| Interstitial fluid † | 0.5 | 9 | 5.5 | 3.2-8.6 |
| Skin | 1 | 10 | 5.3 | 1.3-16.7 |
| Interstitial fluid † | 1 | 5 | 26.3 | 20.9-37.4 |
| Colon | 1 | 2 | 2.6 | 2.5-2.7 |
| Bile | 1 | 7 | 14.6 (3 hours) | 4-25.7 |
| Gall bladder | 1 | 1 | — | 3.9 |
| Peritoneal fluid | 1 | 9 | 30.2 | 7.4-54.6 |
| Lung | 1 | 2 | 4.8 (2 hours) | 1.4-8.2 |
| Bronchial mucosa | 1 | 7 | 4.5 | 1.3-11.1 |
| Muscle | 1 | 2 | 6.1 (2 hours) | 5.3-6.9 |
| Fascia | 1 | 9 | 8.8 | 1.5-20 |
| Heart valves | 1 | 7 | 9.7 | 6.4-12.1 |
| Myocardium | 1 | 10 | 15.5 | 5.2-25.5 |
| CSF (inflamed) | 20 mg/kg
‡
40 mg/kg § | 8
5 | 1.1 (2 hours)
3.3 (3 hours) | 0.2-2.8
0.9-6.5 |
| CSF (uninflamed) | 1 | 4 | 0.2 (2 hours) | 0.1-0.3 |
Elimination
In subjects with normal renal function, the elimination half-life of meropenem is approximately 1 hour.
Excretion
Meropenem is primarily excreted unchanged by the kidneys. Approximately 70% (50% - 75%) of the dose is excreted unchanged within 12 hours. A further 28% is recovered as the microbiologically inactive metabolite. Fecal elimination represents only approximately 2% of the dose. The measured renal clearance and the effect of probenecid show that meropenem undergoes both filtration and tubular secretion. Urinary concentrations of meropenem in excess of 10 mcg/mL are maintained for up to 5 hours after a 500 mg dose.
Specific Populations
Patients with Renal Impairment
Pharmacokinetic studies with meropenem in patients with renal impairment have shown that the plasma clearance of meropenem correlates with creatinine clearance. Dosage adjustments are necessary in subjects with renal impairment (creatinine clearance 50 mL/min or less) [see Dosage and Administration (2.2), Use In Specific Populations (8.6)].
Meropenem is hemodialyzable. However, there is no information on the usefulness of hemodialysis to treat overdosage [see Overdosage (10)].
Patients with Hepatic Impairment
A pharmacokinetic study with meropenem in patients with hepatic impairment has shown no effects of liver disease on the pharmacokinetics of meropenem.
Geriatric Patients
A pharmacokinetic study with meropenem in elderly patients with renal impairment showed a reduction in plasma clearance of meropenem that correlates with age-associated reduction in creatinine clearance.
Pediatric Patients
The pharmacokinetics of meropenem, in pediatric patients 2 years of age or older, are similar to those in adults. The elimination half-life for meropenem was approximately 1.5 hours in pediatric patients of age 3 months to 2 years.
The pharmacokinetics of meropenem in patients less than 3 months of age receiving combination antibacterial drug therapy are given below.
Table 6: Meropenem Pharmacokinetic Parameters in Patients Less Than 3 Months of Age
1
| GA less than 32 weeks PNA less than 2 weeks
(20mg/kg every 12 hours) | GA less than 32 weeks PNA 2 weeks or older
(20mg/kg every 8 hours) | GA 32 weeks or older PNA less than 2 weeks
(20mg/kg every 8 hours) | GA 32 weeks or older PNA 2 weeks or older
(30mg/kg every 8 hours) | Overall | |
|---|---|---|---|---|---|
| CL (L/h/kg) | 0.089 | 0.122 | 0.135 | 0.202 | 0.119 |
| V (L/kg) | 0.489 | 0.467 | 0.463 | 0.451 | 0.468 |
| AUC 0-24(mcg-h/mL) | 448 | 491 | 445 | 444 | 467 |
| C max(mcg/mL) | 44.3 | 46.5 | 44.9 | 61 | 46.9 |
| C min(mcg/mL) | 5.36 | 6.65 | 4.84 | 2.1 | 5.65 |
| T1/2 (h) | 3.82 | 2.68 | 2.33 | 1.58 | 2.68 |
- 1
- Values are derived from a population pharmacokinetic analysis of sparse data
Drug Interactions
Probenecid competes with meropenem for active tubular secretion and thus inhibits the renal excretion of meropenem. Following administration of probenecid with meropenem, the mean systemic exposure increased 56% and the mean elimination half-life increased 38% [see Drug Interactions (7.1)].
12.4 Microbiology
Mechanism of Action
The bactericidal activity of meropenem results from the inhibition of cell wall synthesis. Meropenem readily penetrates the cell wall of most gram-positive and gram-negative bacteria to bind penicillin-bindingprotein (PBP) targets. Meropenem binds to PBPs 2, 3 and 4 of Escherichia coli and Pseudomonas aeruginosa; and PBPs 1, 2 and 4 of Staphylococcus aureus.Bactericidal concentrations (defined as a 3 log 10reduction in cell counts within 12 hours to 24 hours) are typically 1to 2 times the bacteriostatic concentrations of meropenem, with the exception of Listeria monocytogenes, against which lethal activity is not observed.
Meropenem does not have in vitroactivity against methicillin-resistant Staphylococcus aureus(MRSA) or methicillin-resistant Staphylococcus epidermidis(MRSE).
Resistance
There are several mechanisms of resistance to carbapenems: 1) decreased permeability of the outer membrane of gram-negative bacteria (due to diminished production of porins) causing reduced bacterial uptake, 2) reduced affinity of the target PBPs, 3) increased expression of efflux pump components, and 4) production of antibacterial drug-destroying enzymes (carbapenemases, metallo-β-lactamases).
Interaction with Other Antimicrobials
In vitrotests show meropenem to act synergistically with aminoglycoside antibacterial drugs against some isolates of Pseudomonas aeruginosa.
Antimicrobial Activity
Meropenem has been shown to be active against most isolates of the following microorganisms, both in vitroand in clinical infections [see Indications and Usage (1)].
Gram-positive bacteria
Enterococcus faecalis(vancomycin-susceptible isolates only)
Staphylococcus aureus(methicillin-susceptible isolates only)
Streptococcus agalactiae
Streptococcus pneumoniae(penicillin-susceptible isolates only)
Streptococcus pyogenes
Viridans group streptococci
| Gram-negative bacteria
Escherichia coli Haemophilus influenzae Klebsiella pneumoniae Neisseria meningitidis Proteus mirabilis Pseudomonas aeruginosa | Anaerobic bacteria
Bacteroides fragilis Bacteroides thetaiotaomicron Peptostreptococcus species |
The following in vitrodata are available, but their clinical significance is unknown. At least 90% of the following bacteria exhibit an in vitrominimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for meropenem against isolates of similar genus or organism group. However, the efficacy of meropenem in treating clinical infections caused by these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria
Staphylococcus epidermidis(methicillin-susceptible isolates only)
| Gram-negative bacteria | |
| Aeromonas hydrophila
Campylobacter jejuni Citrobacter freundii Citrobacter koseri Enterobacter cloacae Hafnia alvei | Klebsiella oxytoca
Moraxella catarrhalis Morganella morganii Pasteurella multocida Proteus vulgaris Serratia marcescens |
| Anaerobic bacteria | |
| Bacteroides ovatus
Bacteroides uniformis Bacteroides ureolyticus Bacteroides vulgatus Clostridium difficile Clostridium perfringens Eggerthella lenta | Fusobacteriumspecies
Parabacteroides distasonis Porphyromonas asaccharolytica Prevotella bivia Prevotella intermedia Prevotella melaninogenica Propionibacterium acnes |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis:
Genetic toxicity studies were performed with meropenem using the bacterial reverse mutation test, the Chinese hamster ovary HGPRT assay, cultured human lymphocytes cytogenic assay, and the mouse micronucleus test. There was no evidence of mutagenic potential found in any of these tests.
Impairment of Fertility:
In fertility studies, intravenous meropenem was administered to male rats beginning 11 weeks before mating and throughout mating and to female rats from 2 weeks before mating through Gestation Day 7 at doses of 240, 500, and 1000 mg/kg/day. There was no evidence of impaired fertility at doses up to 1000 mg/kg/day (on the basis of body surface area comparison, approximately 3.2 times to the MRHD of 1 gram every 8 hours).
14 CLINICAL STUDIES
14.1 Complicated Skin and Skin Structure Infections
Adult patients with complicated skin and skin structure infections including complicated cellulitis, complex abscesses, perirectal abscesses, and skin infections requiring intravenous antimicrobials, hospitalization, and surgical intervention were enrolled in a randomized, multi-center, international, double-blind trial. The study evaluated meropenem at doses of 500 mg administered intravenously every 8 hours and imipenem-cilastatin at doses of 500 mg administered intravenously every 8 hours. The study compared the clinical response between treatment groups in the clinically evaluable population at the follow-up visit (test-of-cure). The trial was conducted in the United States, South Africa, Canada, and Brazil. At enrollment, approximately 37% of the patients had underlying diabetes, 12% had underlying peripheral vascular disease and 67% had a surgical intervention. The study included 510 patients randomized to meropenem and 527 patients randomized to imipenem-cilastatin. Two hundred and sixty one (261) patients randomized to meropenem and 287 patients randomized to imipenem-cilastatin were clinically evaluable. The success rates in the clinically evaluable patients at the follow-up visit were 86% (225/261) in the meropenem arm and 83% (238/287) in imipenem-cilastatin arm.
The success rates for the clinically evaluable population are provided in Table 7.
Table 7: Success Rates at Test-of-Cure Visit for Clinically Evaluable Population with Complicated Skin and Skin Structure Infections
| Population | Meropenem n */N †(%) | Imipenem-cilastatin n */N †(%) |
|---|---|---|
| Total | 225/261 (86) | 238/287 (83) |
| Diabetes mellitus | 83/97 (86) | 76/105 (72) |
| No diabetes mellitus | 142/164 (87) | 162/182 (89) |
| Less than 65 years of age | 190/218 (87) | 205/241 (85) |
| 65 years of age or older | 35/43 (81) | 33/46 (72) |
| Men | 130/148 (88) | 137/172 (80) |
| Women | 95/113 (84) | 101/115 (88) |
The clinical efficacy rates by pathogen are provided in Table 8. The values represent the number of patients clinically cured/number of clinically evaluable patients at the post-treatment follow-up visit, with the percent cure in parentheses (Fully Evaluable analysis set).
Table 8: Clinical Efficacy Rate by Pathogen for Clinically Evaluable Population
| MICROORGANISMS * | Meropenem n †/N ‡(%) § | Imipenem-cilastatin n †/N ‡(%) § |
|---|---|---|
| Gram-positive aerobes | ||
| Staphylococcus aureus,methicillin susceptible | 82/88 (93) | 84/100 (84) |
| Streptococcus pyogenes(Group A) | 26/29 (90) | 28/32 (88) |
| Streptococcus agalactiae(Group B) | 12/17 (71) | 16/19 (84) |
| Enterococcus faecalis | 9/12 (75) | 14/20 (70) |
| Viridans group streptococci | 11/12 (92) | 5/6 (83) |
| Gram-negative aerobes | ||
| Escherichia coli | 12/15 (80) | 15/21 (71) |
| Pseudomonas aeruginosa | 11/15 (73) | 13/15 (87) |
| Proteus mirabilis | 11/13 (85) | 6/7 (86) |
| Anaerobes | ||
| Bacteroides fragilis | 10/11 (91) | 9/10 (90) |
| Peptostreptococcusspecies | 10/13 (77) | 14/16 (88) |
The proportion of patients who discontinued study treatment due to an adverse event was similar for both treatment groups (meropenem, 2.5% and imipenem-cilastatin, 2.7%).
14.2 Complicated Intra-Abdominal Infections
One controlled clinical study of complicated intra-abdominal infection was performed in the United States where meropenem was compared with clindamycin/tobramycin. Three controlled clinical studies of complicated intra-abdominal infections were performed in Europe; meropenem was compared with imipenem (two trials) and cefotaxime/metronidazole (one trial).
Using strict evaluability criteria and microbiologic eradication and clinical cures at follow-up which occurred 7 or more days after completion of therapy, the presumptive microbiologic eradication/clinical cure rates and statistical findings are provided in Table 9:
Table 9: Presumptive Microbiologic Eradication and Clinical Cure Rates at Test-of-Cure Visit in the Evaluable Population with Complicated Intra-Abdominal Infection
| Treatment Arm | No. evaluable/No. enrolled
(%) | Microbiologic Eradication Rate | Clinical Cure Rate | Outcome |
|---|---|---|---|---|
| meropenem | 146/516
(28%) | 98/146
(67%) | 101/146
(69%) | |
| imipenem | 65/220
(30%) | 40/65
(62%) | 42/65
(65%) | meropenem equivalent to control |
| cefotaxime/metronidazole | 26/85
(30%) | 22/26
(85%) | 22/26
(85%) | meropenem not equivalent to control |
| clindamycin/tobramycin | 50/212
(24%) | 38/50
(76%) | 38/50
(76%) | meropenem equivalent to control |
The finding that meropenem was not statistically equivalent to cefotaxime/metronidazole may have been due to uneven assignment of more seriously ill patients to the meropenem arm. Currently there is no additional information available to further interpret this observation.
14.3 Bacterial Meningitis
Four hundred forty-six patients (397 pediatric patients 3 months to less than 17 years of age) were enrolled in 4 separate clinical trials and randomized to treatment with meropenem (n=225) at a dose of 40 mg/kg every 8 hours or a comparator drug, i.e., cefotaxime (n=187) or ceftriaxone (n=34), at the approved dosing regimens. A comparable number of patients were found to be clinically evaluable (ranging from 61-68%) and with a similar distribution of pathogens isolated on initial CSF culture.
Patients were defined as clinically not cured if any one of the following three criteria were met:
- At the 5 to 7 week post-completion of therapy visit, the patient had any one of the following: moderate to severe motor, behavior or development deficits, hearing loss of greater than 60 decibels in one or both ears, or blindness.
- During therapy the patient's clinical status necessitated the addition of other antibacterial drugs.
- Either during or post-therapy, the patient developed a large subdural effusion needing surgical drainage, or a cerebral abscess, or a bacteriologic relapse.
Using the definition, the following efficacy rates were obtained, per organism (noted in Table 10). The values represent the number of patients clinically cured/number of clinically evaluable patients, with the percent cure in parentheses.
Table 10: Efficacy rates by Pathogen in the Clinically Evaluable Population with Bacterial Meningitis
| MICROORGANISMS | Meropenem | COMPARATOR |
|---|---|---|
| S. pneumoniae | 17/24 (71) | 19/30 (63) |
| H. influenzae(+) * | 8/10 (80) | 6/6 (100) |
| H. influenzae(-/NT) † | 44/59 (75) | 44/60 (73) |
| N. meningitidis | 30/35 (86) | 35/39 (90) |
| Total (including others) | 102/131 (78) | 108/140 (77) |
Sequelae were the most common reason patients were assessed as clinically not cured.
Five patients were found to be bacteriologically not cured, 3 in the comparator group (1 relapse and 2 patients with cerebral abscesses) and 2 in the meropenem group (1 relapse and 1 with continued growth of Pseudomonas aeruginosa).
With respect to hearing loss, 263 of the 271 evaluable patients had at least one hearing test performed post-therapy. The following Table 11 shows the degree of hearing loss between the meropenem-treated patients and the comparator-treated patients.
Table 11: Hearing Loss at Post-Therapy in the Evaluable Population Treated with Meropenem
| Degree of Hearing Loss (in one or both ears) | Meropenem
n = 128 | Comparator
n = 135 |
|---|---|---|
| No loss | 61% | 56% |
| 20-40 decibels | 20% | 24% |
| Greater than 40-60 decibels | 8% | 7% |
| Greater than 60 decibels | 9% | 10% |
15 REFERENCES
- Cockcroft DW, MH Gault, 1976, Prediction of creatinine clearance from serum creatinine, Nephron, 16:31-41.
- Kawamura S, AW Russell, SJ Freeman, and RA Siddall, 1992, Reproductive and Developmental Toxicity of Meropenem in Rats, Chemotherapy, 40:S238-250.
16 HOW SUPPLIED/STORAGE AND HANDLING
Meropenem for injection is supplied in 20 mL and 30 mL injection vials containing sufficient meropenem to deliver 500 mg or 1 gram for intravenous administration, respectively. The dry powder should be stored at controlled room temperature 20º to 25ºC (68º to 77ºF) [see USP].
500 mg Injection Vial (NDC 0781-3000-94) and packaged in cartons of 10 vials (NDC 0781-3000-95) and cartons of 25 vials (NDC 0781-3000-96).
1 gram Injection Vial (NDC 0781-3098-94) and packaged in cartons of 10 vials (NDC 0781-3098-95) and cartons of 25 vials (NDC 0781-3098-96).
17 PATIENT COUNSELING INFORMATION
- Counsel patients that antibacterial drugs including meropenem for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When meropenem for injection is prescribed to treat a bacterial infection, tell patients that although it is common to feel better early in the course of therapy, take the medication exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by meropenem for injection or other antibacterial drugs in the future.
- Counsel patients that diarrhea is a common problem caused by antibacterial drugs which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible [see Warnings and Precautions (5.5)].
- Counsel patients to inform their physician if they are taking valproic acid or divalproex sodium. Valproic acid concentrations in the blood may drop below the therapeutic range upon co-administration with meropenem for injection. If treatment with meropenem for injection is necessary and continued, alternative or supplemental anti-convulsant medication to prevent and/or treat seizures may be needed [see Warnings and Precautions (5.4)].
- Patients receiving meropenem for injection on an outpatient basis must be alerted of adverse events such as seizures, delirium, headaches and/or paresthesias that could interfere with mental alertness and/or cause motor impairment. Until it is reasonably well established that meropenem for injection is well tolerated, patients should not operate machinery or motorized vehicles [see Warnings and Precautions (5.9)].
Manufactured by:
Savior Lifetec Corporation
4F., No. 12 & 16, Chuangye Rd., Xinshi Dist., Tainan City 74144, Taiwan for
Sandoz Inc., Princeton, NJ 08540
Rx only
Revised: 12/2019
PRINCIPAL DISPLAY PANEL - 500 mg Vial Carton
NDC0781-3000-95
Meropenem for Injection, USP
500 mg per vial
Meropenem Equivalent
For Intravenous Use Only
Discard unused portion
Rx only
10 x 500 mg Single Dose Vials
SANDOZ
A Novartis Division

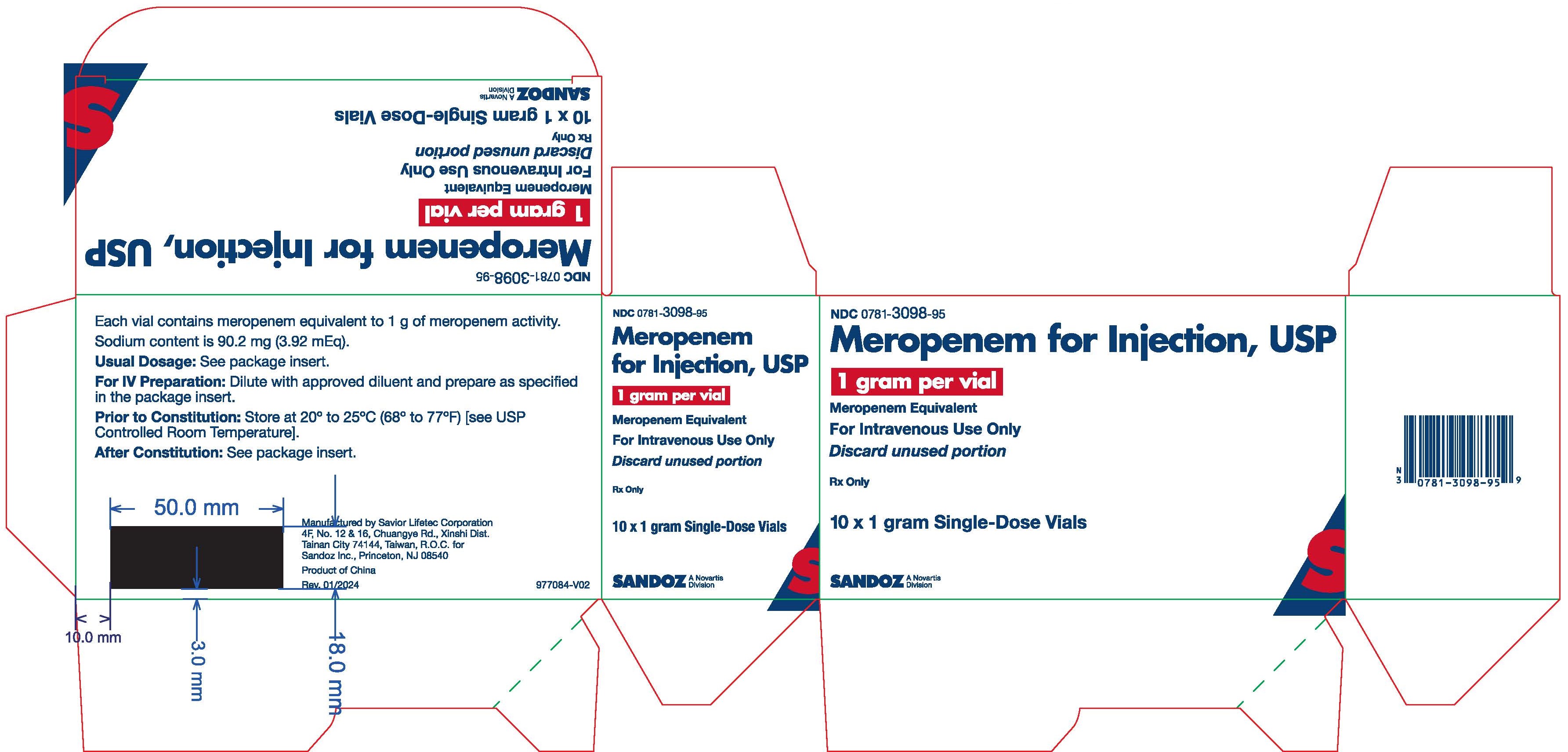
PRINCIPAL DISPLAY PANEL - 1 gram Vial Carton
NDC0781-3098-95
Meropenem for Injection, USP
1 gram per vial
Meropenem Equivalent
For Intravenous Use Only
Discard unused portion
Rx only
10 x 1 gram Single Dose Vials
SANDOZ
A Novartis Division