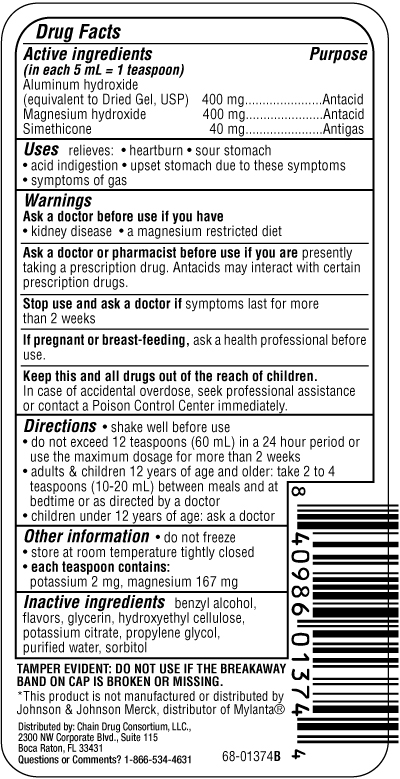
Active ingredients
Aluminum hydroxide(equivalent to Dried Gel, USP) 400 mg
Magnesium hydroxide 400 mg
Simethicone 40 mg
Uses
relieves: • heartburn • sour stomach • acid indigestion
• upset stomach due to these symptoms • symptoms of gas
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain
prescription drugs
Keep this and all drugs out of the reach of children.
In case of accidental overdose, seek professional assistance
or contact a Poison Control Center immediately.
Other information
store at room temperature tightly closed
do not freeze
each teaspoon contains:
potassium 2 mg, magnesium 167 mg
Inactive ingredients
benzyl alcohol,
flavors, glycerin, hydroxyethyl cellulose,
potassium citrate, propylene glycol,
purified water, sorbitol
Directions
- shake well before use
- do not exceed 12 teaspoons (60 mL) in a 24 hour period or use the maximum dosage for more than 2 weeks
- adults and children 12 years of age and older: take 2 to 4 teaspoons (10-20 mL) between meals and at bedtime or as directed by a doctor
- children under 12 years of age: ask a doctor