Uses
For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises, and sprains.
Warnings
For external use only
When using this product: • use only as directed • do not apply to wounds or damaged skin • avoid contact with eyes • do not bandage tightly
Stop use and ask a doctor if: condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: Consult a doctor.
- Clean skin of oil, dirt, lotions, etc. and dry completely before use.
- If necessary, use medical tape, tension bandage or sleeve to secure in place.
Inactive ingredients
activated charcoal, alcohol, borneol, dihydroxyaluminum aminoacetic acid, disodium EDTA, DMDM ethyl urea, glycerin, polyvinyl pyrrolidone povidone K30, sodium polyacrylate, water
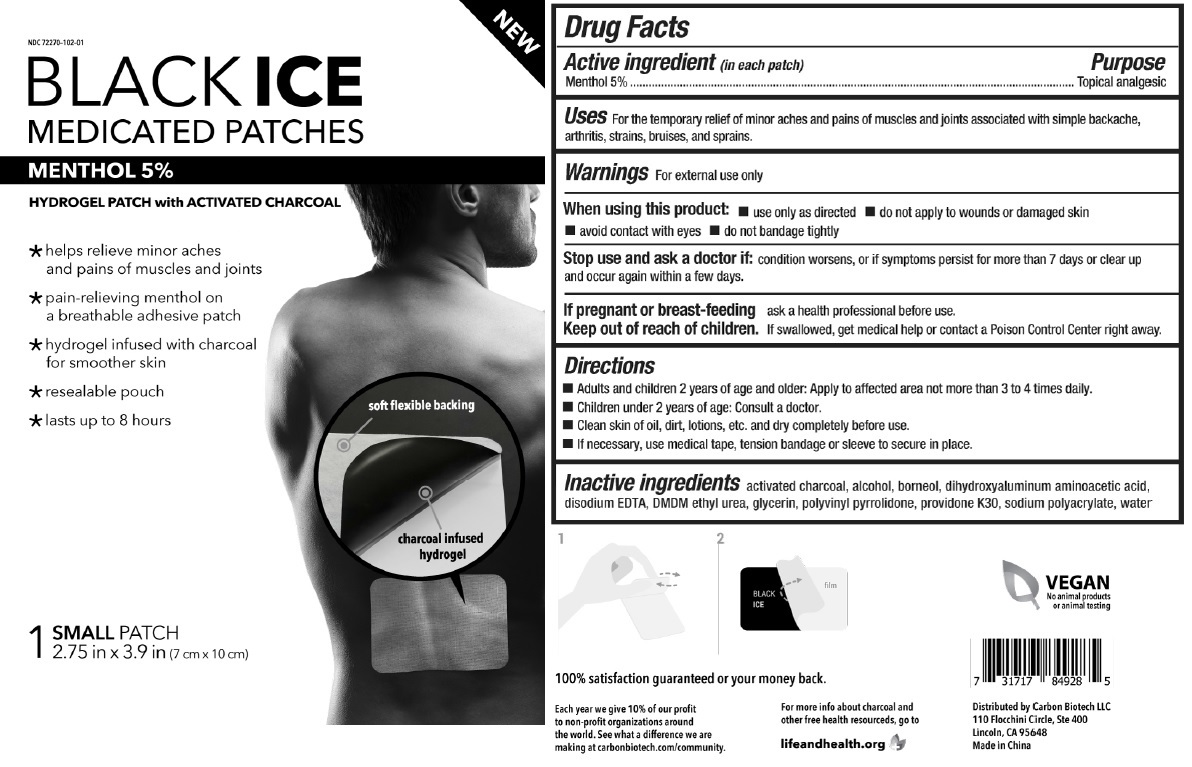
HYDROGEL PATCH with ACTIVATED CHARCOAL
* helps relieve minor aches and pains of muscles and joints
* pain-relieving menthol on a breathable adhesive patch
* hydrogel infused with charcoal for smoother skin
* resealable pouch
* lasts up to 8 hours
VEGAN
No animal products or animal testing
For more info about charcoal and other free health resources, go to lifeandhealth.org
Distributed by Carbon Biotech LLC
110 Flocchini Circle, Ste 400
Lincoln, CA 95648
Made in China