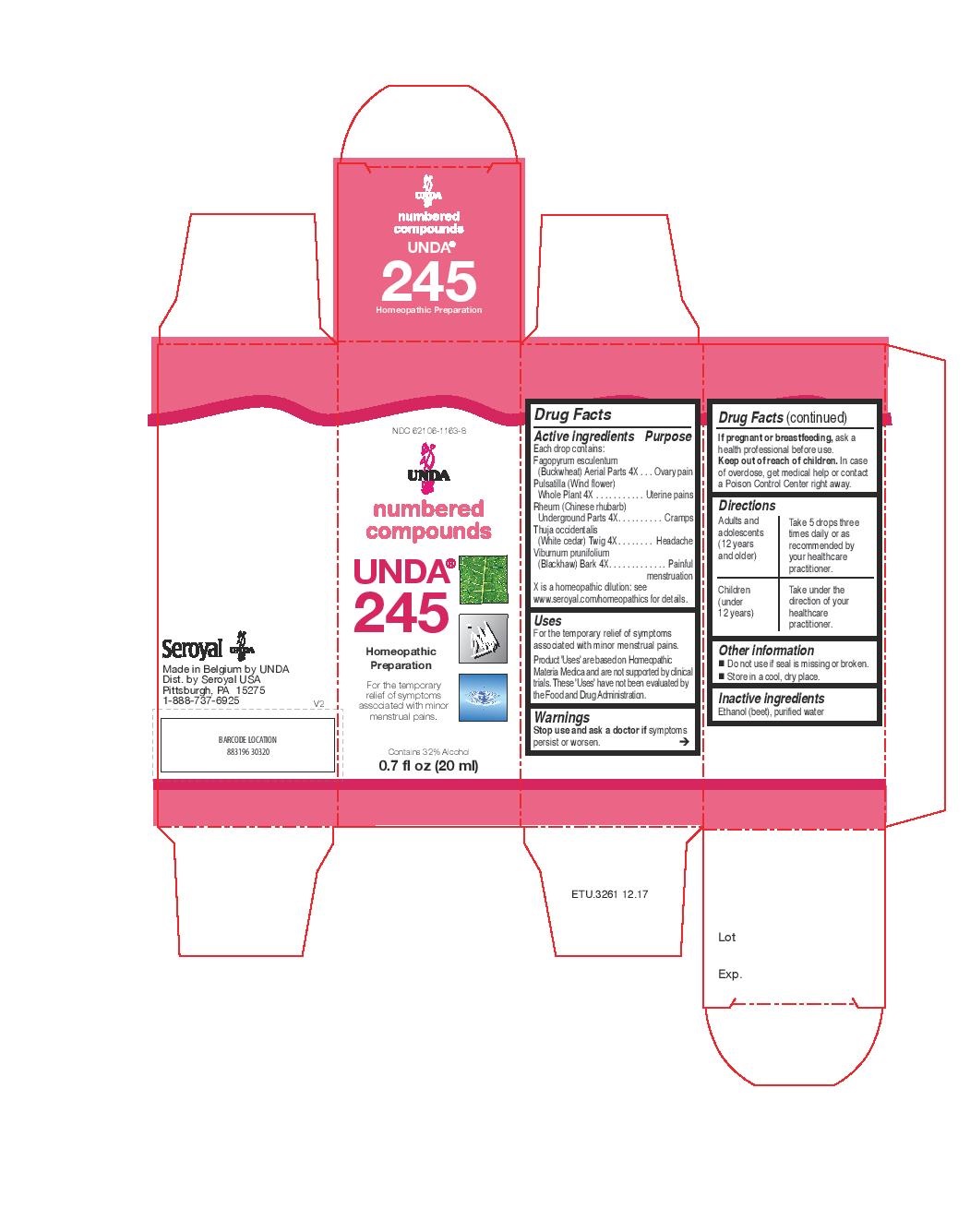
Active ingredients
Each drop contains:
Fagopyrum esculentum (Buckwheat) Aerial Parts 4X
Pulsatilla (Wind flower) Whole Plant 4X
Rheum (Chinese rhubarb) Underground Parts 4X
Thuja occidentalis (White cedar) Twig 4X
Viburnum prunifolium (Blackhaw) Bark 4X
Warnings
Stop use and ask a doctor if symptoms
persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with minor menstrual pains.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.