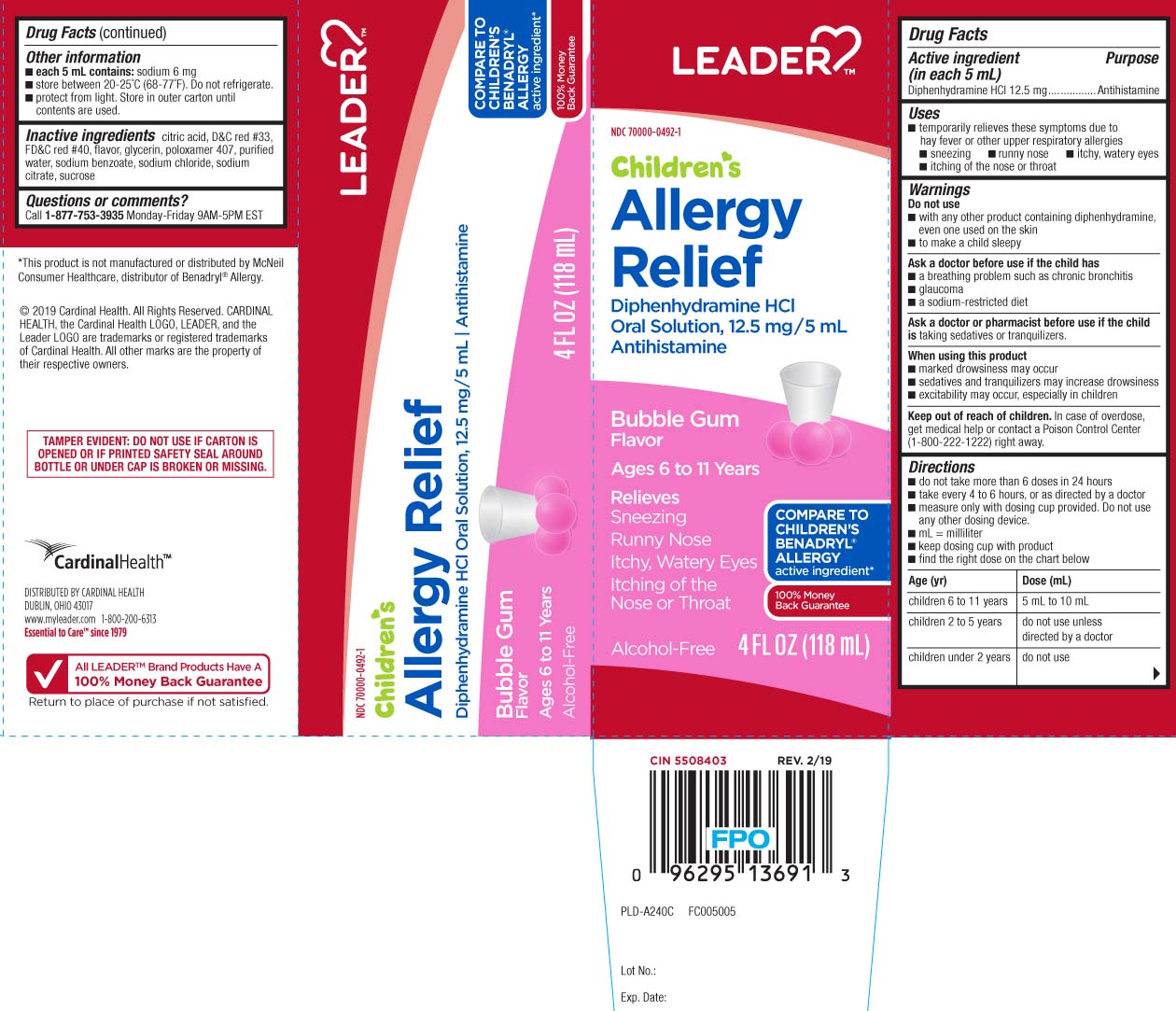
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- runny nose
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if the child has
- a breathing problem such as chronic bronchitis
- glaucoma
- a sodium-restricted diet
Directions
- do not take more than 6 doses in 24 hours
- take every 4 to 6 hours, or as directed by a doctor
- measure only with dosing cup provided. Do not use any other dosing device.
- mL = milliliter
- keep dosing cup with product
- find the right dose on the chart below
| age (yr) | dose (mL) |
| children 6 to 11 years | 5 mL to 10 mL |
| children 2 to 5 years | do not use unless directed by a doctor |
| children under 2 years | do not use |
Other information
- each 5 mL contains: sodium 6 mg
- store between 20-25ºC (68-77ºF). Do not refrigerate
- Protect from light. Store in outer carton until contents are used
Inactive ingredients
anhydrous citric acid, D&C red #33, RD&C red#40, flavors, glycerin, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucrose
Principal Display Panel
COMPARE TO CHILDREN'S BENADRYL® ALLERGY active ingredient*
Children's
Allergy Relief
Diphenhydramine HCI
Oral Solution, 12.5 mg/5mL
Antihistamine
Bubble Gum Flavor
Ages 6 to 11 years
Relieves
Sneezing
Runny Nose
Itchy, Watery Eyes
Itching of the Nose or Throat
Alcohol-Free
Dosing Cup included
FL OZ (mL)
*This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Benadryl® Allergy
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017