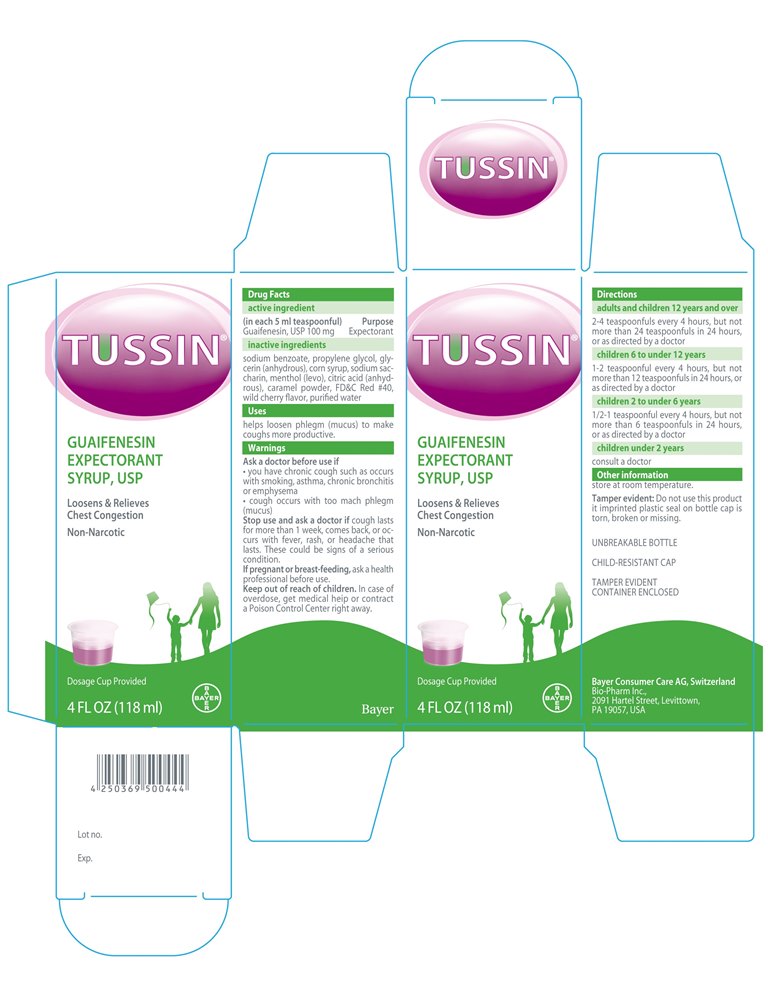
Ask a doctor before use if
- you have chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough occurs with too much mach phlegm (mucus)
Stop use and ask a doctor if cough lasts for more than 1 week, comes back, or occurs with fever, rash, or headache that lasts. These could be signs of a seriuos conditions.