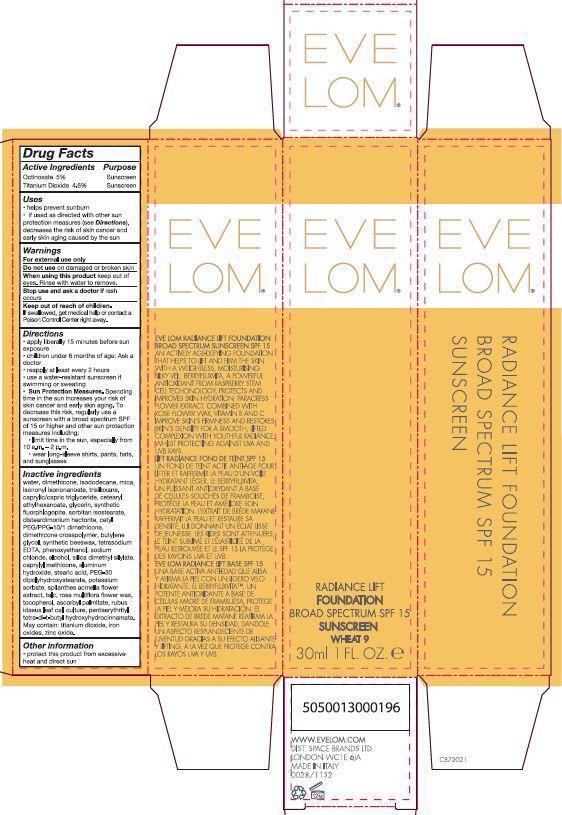
Uses
- helps prevent sunburn
- if used as directed with other sun protection measure (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- children under 6 months of age: Ask a doctor
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
water, dimethicone, isododecane, mica, isononyl isononanoate, trisiloxane, caprylic/capric triglyceride, cetearyl ethlyhexanoate, glycerin, synthetic fluorphlogopite, sorbitan isostearate, disteardimonium hectorite, cetyl PEG/PPG-10/1 dimethicone, dimethicone crosspolymer, butylene glycol, synthetic beeswax, tetrasodium EDTA, phenoxyethanol, sodium chloride, alcohol, silica dimethyl silylate, caprylyl methicone, aluminum hydroxide, stearic acid, PEG-30 dipolyhydroxystearate, potassium sorbate, spilanthes acmella flower extract, talc, rosa multiflora flower wax, tocopherol, ascorbyl palmitate, rubus idaeus leaf cell culture, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate.
May contain: titanium dioxide, iron oxides, zinc oxide.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Ivory
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
IVORY 2
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Ecru
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
ECRU 3
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Vanilla
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
VANILLA 4
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Linen
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
LINEN 5
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Cream
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
CREAM 6
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Beige
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
BEIGE 7
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Petal
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
PETAL 8
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Wheat
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
WHEAT 9
30 ml 1 FL. OZ. e
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Bamboo
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
BAMBOO 10
30 ml 1 FL. OZ. e

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Glow
EVE LOM®
RADIANCE LIFT
FOUNDATION
BROAD SPECTRUM SPF 15
SUNSCREEN
GLOW 11
30 ml 1 FL. OZ. e


