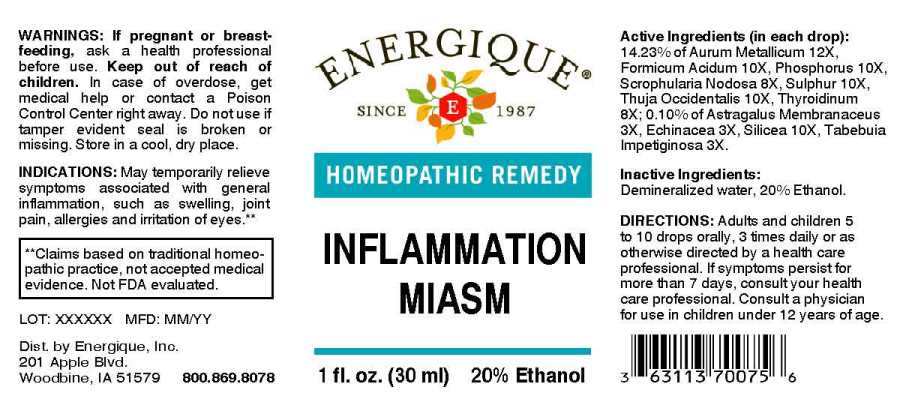
ACTIVE INGREDIENTS:
(in each drop): 14.23% of Aurum Metallicum 12X, Formicum Acidum 10X, Phosphorus 10X, Scrophularia Nodosa 8X, Sulphur 10X, Thuja Occidentalis 10X, Thyroidinum (Suis) 8X; 0.10% of Astragalus Membranaceus 3X, Echinacea (Angustifolia) 3X, Silicea 10X, Tabebuia Impetiginosa 3X.
INDICATIONS:
May temporarily relieve symptoms associated with general inflammation, such as swelling, joint pain, allergies and irritation of eyes.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist for more than 7 days, consult your health care professional. Consult a physician for use in children under 12 years of age.