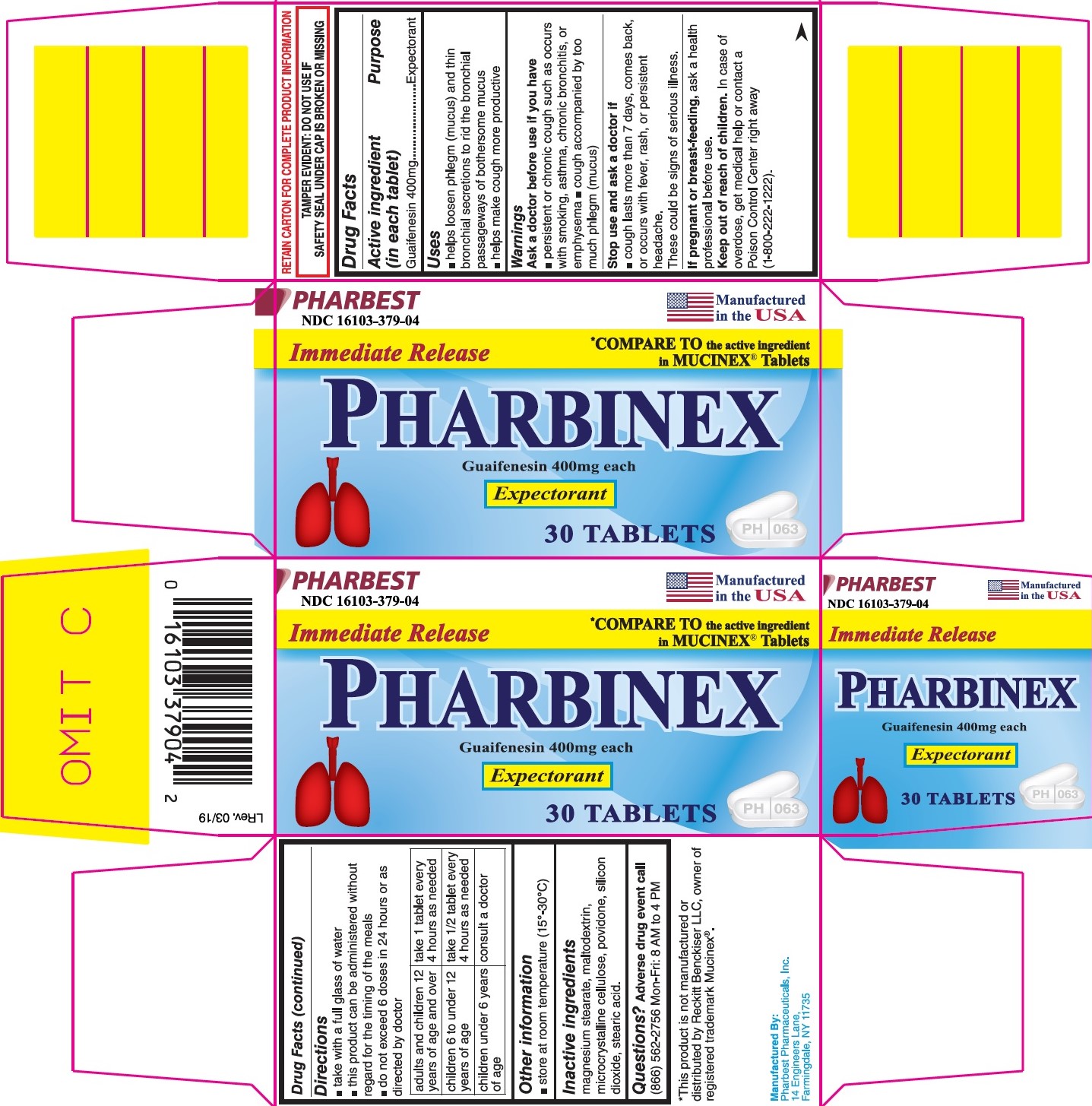
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus
- helps make cough more productive
Warnings
Ask doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache.
These could be signs of serious illness.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- take with a full glass of water
- this product can be administered without regard for the timing of the meals
- do not exceed 6 doses in 24 hours or as directed by doctor
|
adults and children 12 years of age and over |
take 1 tablet every 4 hours as needed |
|
children 6 to under 12 years of age |
take ½ tablet every 4 hours as needed |
|
children under 6 years of age |
consult a doctor |