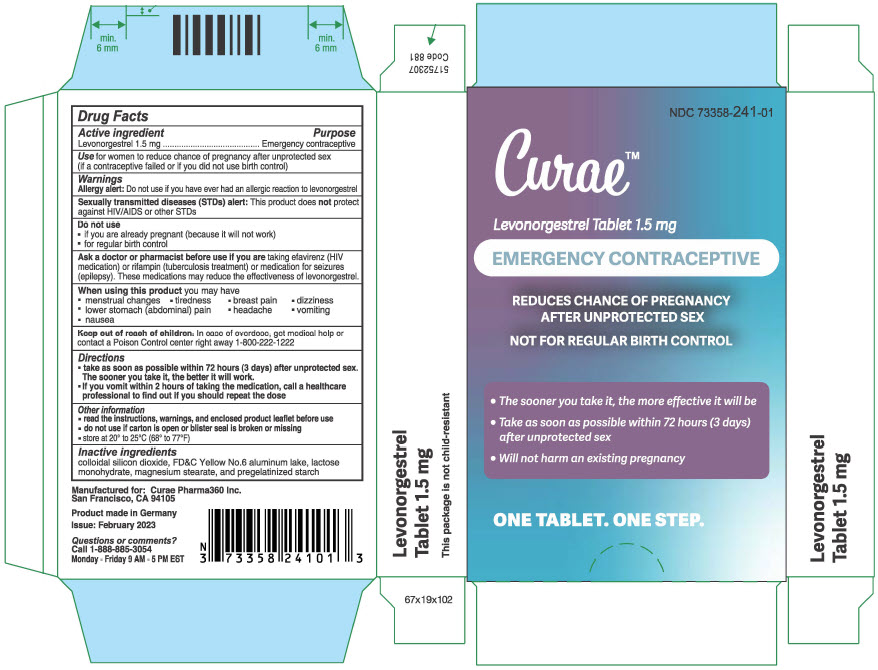
Use
for women to reduce chance of pregnancy after unprotected sex
(if a contraceptive failed or if you did not use birth control)
Warnings
Sexually transmitted diseases (STDs) alert
This product does not protect against HIV/AIDS or other STDs
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
Directions
- take as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it, the better it will work.
- If you vomit within 2 hours of taking the medication, call a healthcare professional to find out if you should repeat the dose
Other information
- read the instructions, warnings, and enclosed product leaflet before use
- do not use if carton is open or blister seal is broken or missing
- store at 20° to 25°C (68° to 77°F)
Inactive ingredients
colloidal silicon dioxide, FD&C Yellow No.6 aluminum lake, lactose monohydrate, magnesium stearate, and pregelatinized starch
Manufactured for:
Curae Pharma360 Inc.
San Francisco, CA 94105
NDC 73358-241-01
Product made in Germany
Issued: March 2023
PRINCIPAL DISPLAY PANEL - 1.5 mg Tablet Blister Pack Carton
NDC 73358-241-01
Curae™
Levonorgestrel Tablet 1.5 mg
EMERGENCY CONTRACEPTIVE
REDUCES CHANCE OF PREGNANCY
AFTER UNPROTECTED SEX
NOT FOR REGULAR BIRTH CONTROL
- The sooner you take it, the more effective it will be
-
Take as soon as possible within 72 hours (3 days)
after unprotected sex - Will not harm an existing pregnancy
ONE TABLET. ONE STEP.