EYE WASH - water solution
Kareway Product, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Purified water (99.05%)
Uses
washes the eye to help relieve
- irritation
- stinging
- discomfort
- itching;
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Warnings
Do not use
- if you have open wounds in or near the eyes, and get medical help right away
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Remove contact lenses before using.
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after use.
------------------------------------------------------------------------------
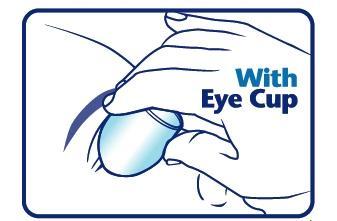
When using an eye cup
- rinse the cup with Pure-aid Eye Wash immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Pure-aid Eye Wash Solution and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse cup with clean water after each use
Other information
- store at room temperature
- keep tightly closed
- use before expiration date marked on the carton or bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride, Hydrochloric acid PRESERVATIVE ADDED: edetate disodium, polyhexamethylene biguanide
Package label
Advanced Eye Wash
Kareway Product, Inc.