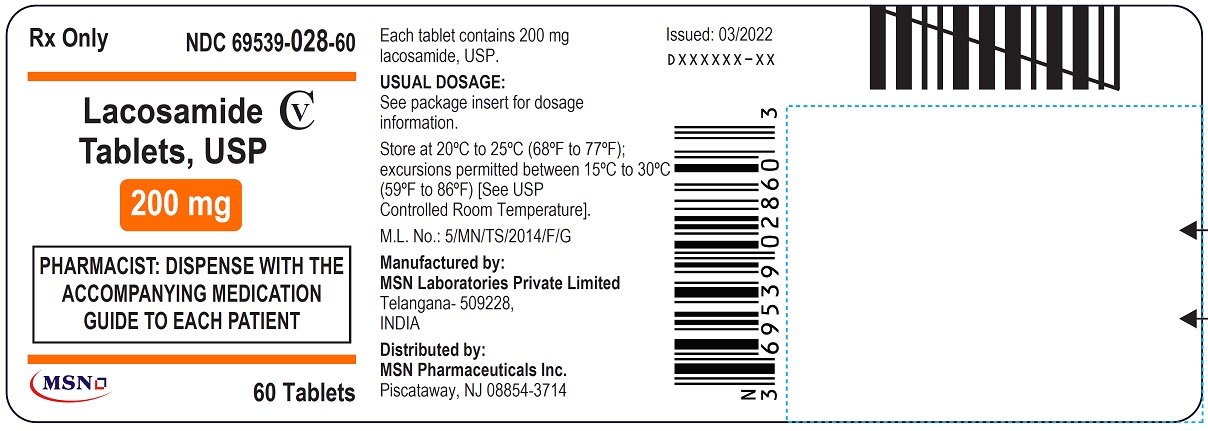
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
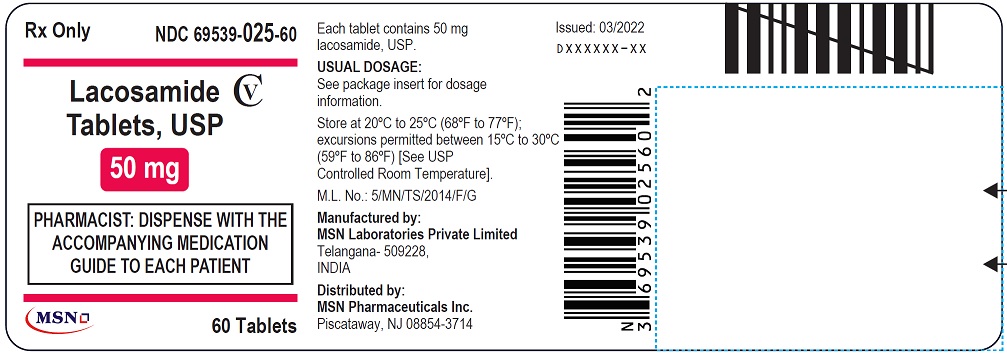
NDC 69539-025-60
Lacosamide Tablet, USP
50 mg
Rx Only
PHARMACIST: DISPENSE WITH THE ACCOMPANYING MEDICATION GUIDE TO EACH PATIENT
60 Tablets
NDC 69539-026-60
Lacosamide Tablet, USP
100 mg
Rx Only
PHARMACIST: DISPENSE WITH THE ACCOMPANYING MEDICATION GUIDE TO EACH PATIENT
60 Tablets

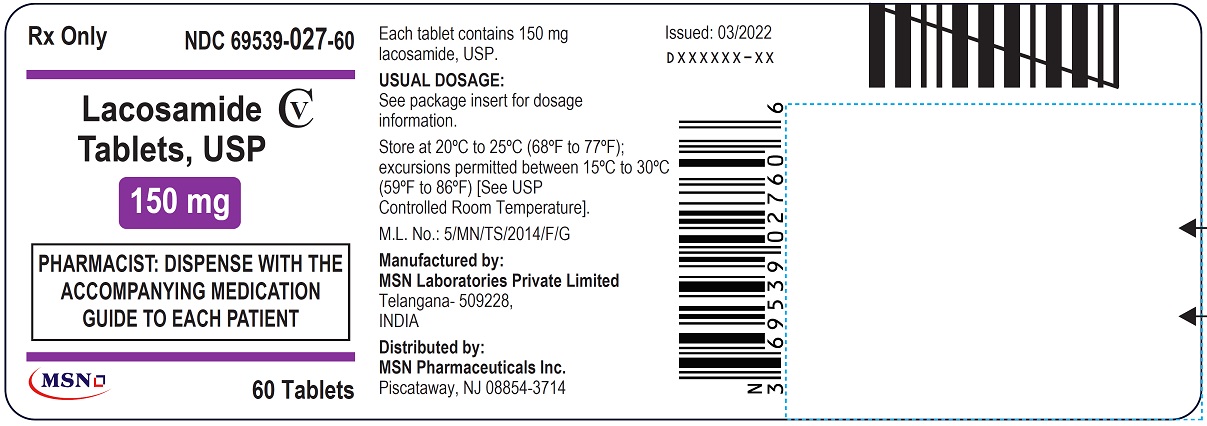
NDC 69539-027-60
Lacosamide Tablet, USP
150 mg
Rx Only
PHARMACIST: DISPENSE WITH THE ACCOMPANYING MEDICATION GUIDE TO EACH PATIENT
60 Tablets
NDC 69539-028-60
Lacosamide Tablet, USP
200 mg
Rx Only
PHARMACIST: DISPENSE WITH THE ACCOMPANYING MEDICATION GUIDE TO EACH PATIENT
60 Tablets