CHAVITA 1- carbo veg, oophorinum, silicea, olibanum, orchitinum, urtica urens liquid
RUBIMED AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
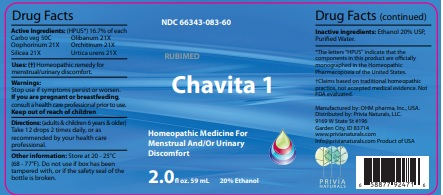
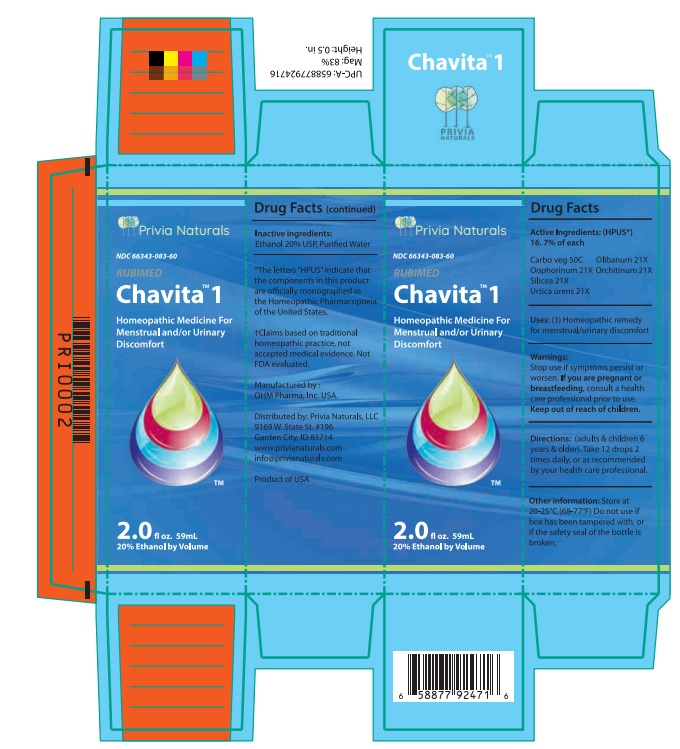
Drug Facts
Active Ingredients: (HPUS*) 16.7% of each
Cargo veg 50C Olibanum 21X
Oophorinum 21X Orchitinum 21X
Silicea 21X Urtica urens 21X
*The letters "HPUS" indicate that the
components in this product are officially
monographed in the Homeopathic
Pharmacopoeia of the United States.
†Claims based on traditional homeopathic
practice, not accepted medical evidence. Not
FDA evaluated.
Uses: (†) Homeopathic remedy for menstrual/urinary discomfort.
Warnings:
Stop use if symptoms persist or worsen.
If you are pregnant or breastfeeding, consult a healthcare professional prior to use.
Keep out of reach of children.
Directions: (adults & children 6 years & older)
Take 12 drops 2 times daily, or as recommended by your health care professional.
Other information: Store at 20 - 25°C
(68 - 77°F.) Do not use if box has been
tampered with, or if the safety seal of the
bottle is broken.
Inactive ingredients: Ethanol 20% USP,
Purified Water.
Manufactured by: OHM pharma, Inc, USA.
Distributed by: Privia Naturals, LLC.
9169 W State St #196
Garden City, ID 83714
www.privianaturals.com
Info@privianaturals.com Product of USA.
NDC 66343-083-60
RUBIMED
Chavita 1
Homeopathic Medicine For
Menstrual And/Or Urinary
Discomfort
2.0 fl oz. 59mL 20% Ethanol
Homeopathic Medicine For
Menstrual And/Or Urinary
Discomfort

