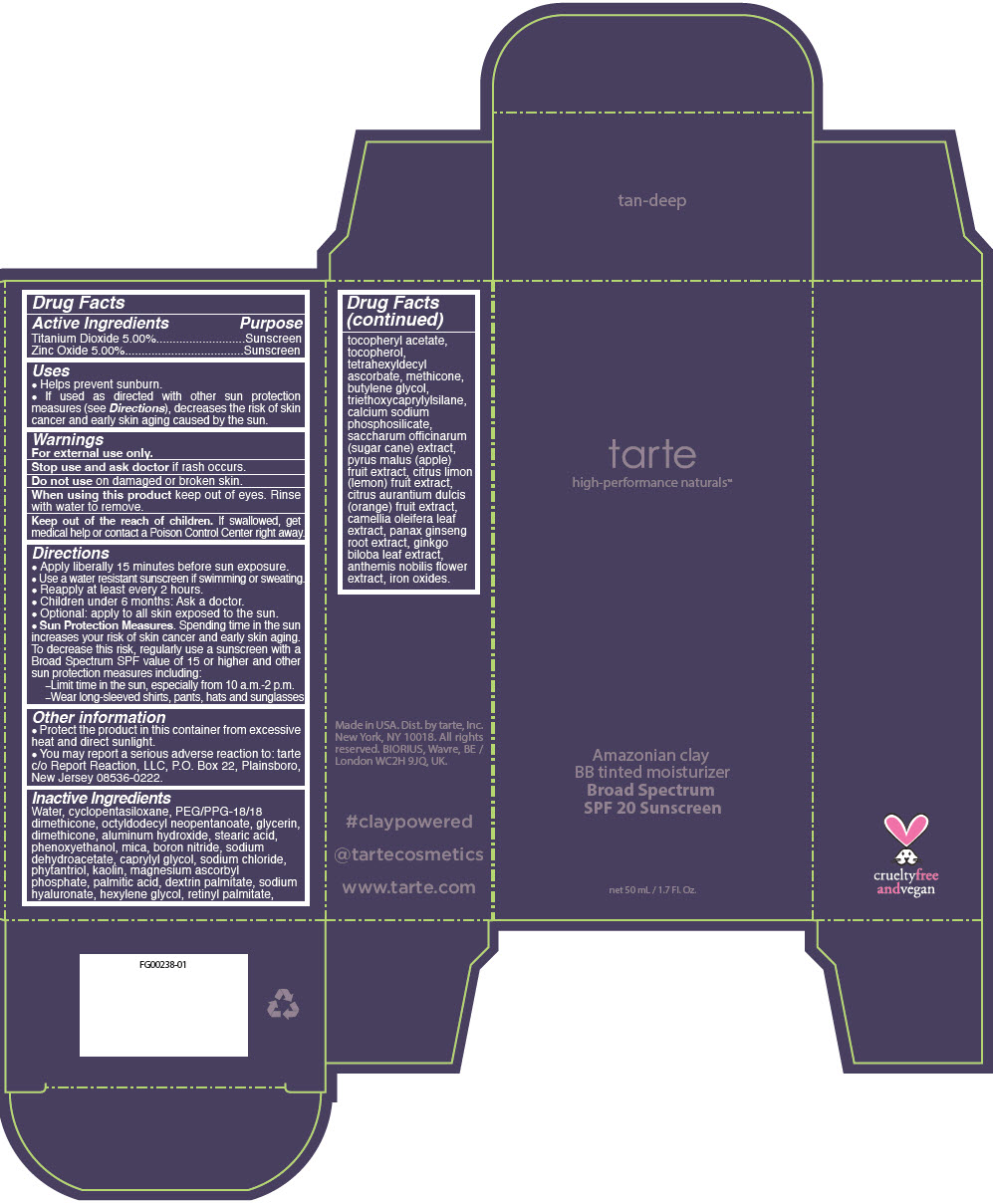
Uses
- helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
- Optional: apply to all skin exposed to the sun
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
Other information
- Protect the product in this container from excessive heat and direct sunlight
- You may report a serious adverse reaction to: tarte c/o Report Reaction, LLC, P.O. Box 22, Plainsboro, New Jersey 08536-0222.
Inactive ingredients
Water, cyclopentasiloxane, PEG/PPG-18/18 dimethicone, octyldodecyl neopentanoate, glycerin, dimethicone, aluminum hydroxide, stearic acid, phenoxyethanol, mica, boron nitride, sodium dehydroacetate, caprylyl glycol, sodium chloride, phytantriol, kaolin, magnesium ascorbyl phosphate, palmitic acid, dextrin palmitate, sodium hyaluronate, hexylene glycol, retinyl palmitate, tocopheryl acetate, tocopherol, tetrahexyldecyl ascorbate, methicone, butylene glycol, triethoxycaprylylsilane, calcium sodium phosphosilicate, saccharum officinarum (sugar cane) extract, pyrus malus (apple) fruit extract, citrus limon (lemon) fruit extract, citrus aurantium dulcis (orange) fruit extract, camellia oleifera leaf extract, panax ginseng root extract, ginkgo biloba leaf extract, anthemis nobilis flower extract, iron oxides.
PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - ivory
tarte
high-performance naturals™
Amazonian clay
BB tinted moisturizer
Broad Spectrum
SPF 20 Sunscreen
net 50 mL / 1.7 Fl. Oz.

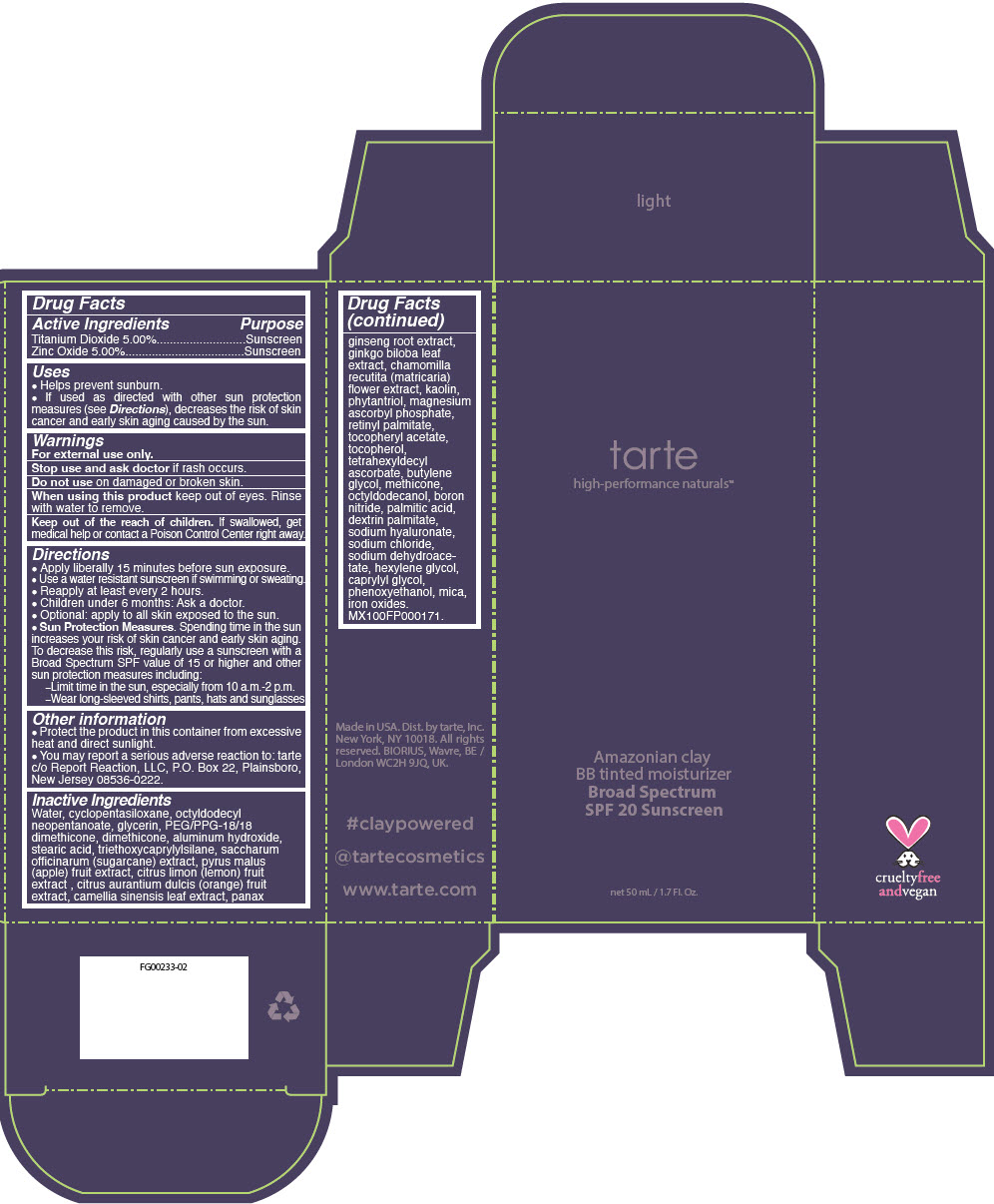
PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - light
tarte
high-performance naturals™
Amazonian clay
BB tinted moisturizer
Broad Spectrum
SPF 20 Sunscreen
net 50 mL / 1.7 Fl. Oz.
PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - light-medium
tarte
high-performance naturals™
Amazonian clay
BB tinted moisturizer
Broad Spectrum
SPF 20 Sunscreen
net 50 mL / 1.7 Fl. Oz.

PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - medium
tarte
high-performance naturals™
Amazonian clay
BB tinted moisturizer
Broad Spectrum
SPF 20 Sunscreen
net 50 mL / 1.7 Fl. Oz.

PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - medium-tan
tarte
high-performance naturals™
Amazonian clay
BB tinted moisturizer
Broad Spectrum
SPF 20 Sunscreen
net 50 mL / 1.7 Fl. Oz.