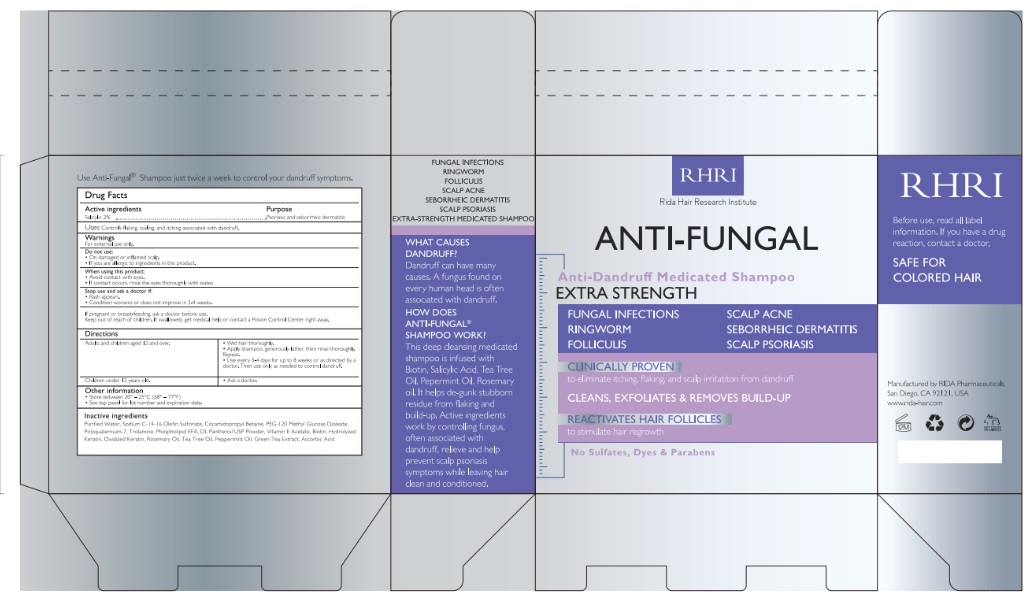
Uses
Relieves and helps prevent recurrence of scalp irritation, flaking , itching, redness , scaling
Warnings
For external use only
Do not use
- On damaged or inflamed scalp
- if you are allergic to ingredients in this product
When using this product
- Avoid contact with eyes
- if contact occurs, rinse the eyes thoroughly with water
Stop use and ask a doctor if
- rash appears
- condition worsens or does not improve in 2-4 weeks.
If pregnant or breast-feeding, ask a doctor use.
Keep out of reach of children. if swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children aged 12 and over :
- wet hair thoroughly
- apply shampoo, generously lather, then rinse thoroughly. Repeate.
- Use every 3-4 days for up to 8 weeks or as direacted by a doctor. Then use only as needed to control dandruff
Cildren under 12 years old
- ask a doctor
Other Information
- store between 20° - 25°C (68° - 77°F)
- see bottom panel for lot number and expiration date