Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
Inactive Ingredients
DIMETHICONE・WATER・GLYCERIN・TRIFLUOROPROPYLDIMETHYL/TRIMETHYLSILOXYSILICATE・PEG-10 DIMETHICONE・BUTYLENE GLYCOL・PHENYL TRIMETHICONE・DIMETHICONE CROSSPOLYMER・BIS-BUTYLDIMETHICONE POLYGLYCERYL-3・SORBITAN SESQUIISOSTEARATE・ISOPROPYL MYRISTATE・DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER・NACRE POWDER・MAGNESIUM ALUMINOMETASILICATE・POLYQUATERNIUM-51・THYMUS SERPYLLUM EXTRACT・SODIUM ACETYLATED HYALURONATE・ALUMINUM HYDROXIDE・STEARIC ACID・CYCLOPENTASILOXANE・DISTEARDIMONIUM HECTORITE・POLYMETHYLSILSESQUIOXANE・POLYSILICONE-2・TRIMETHYLSILOXYSILYLCARBAMOYL PULLULAN・LITHIUM MAGNESIUM SODIUM SILICATE・PEG/PPG-19/19 DIMETHICONE・CITRIC ACID・SODIUM CITRATE・BHT・SILICA・TOCOPHEROL・PHENOXYETHANOL・TITANIUM DIOXIDE・IRON OXIDES・MICA・
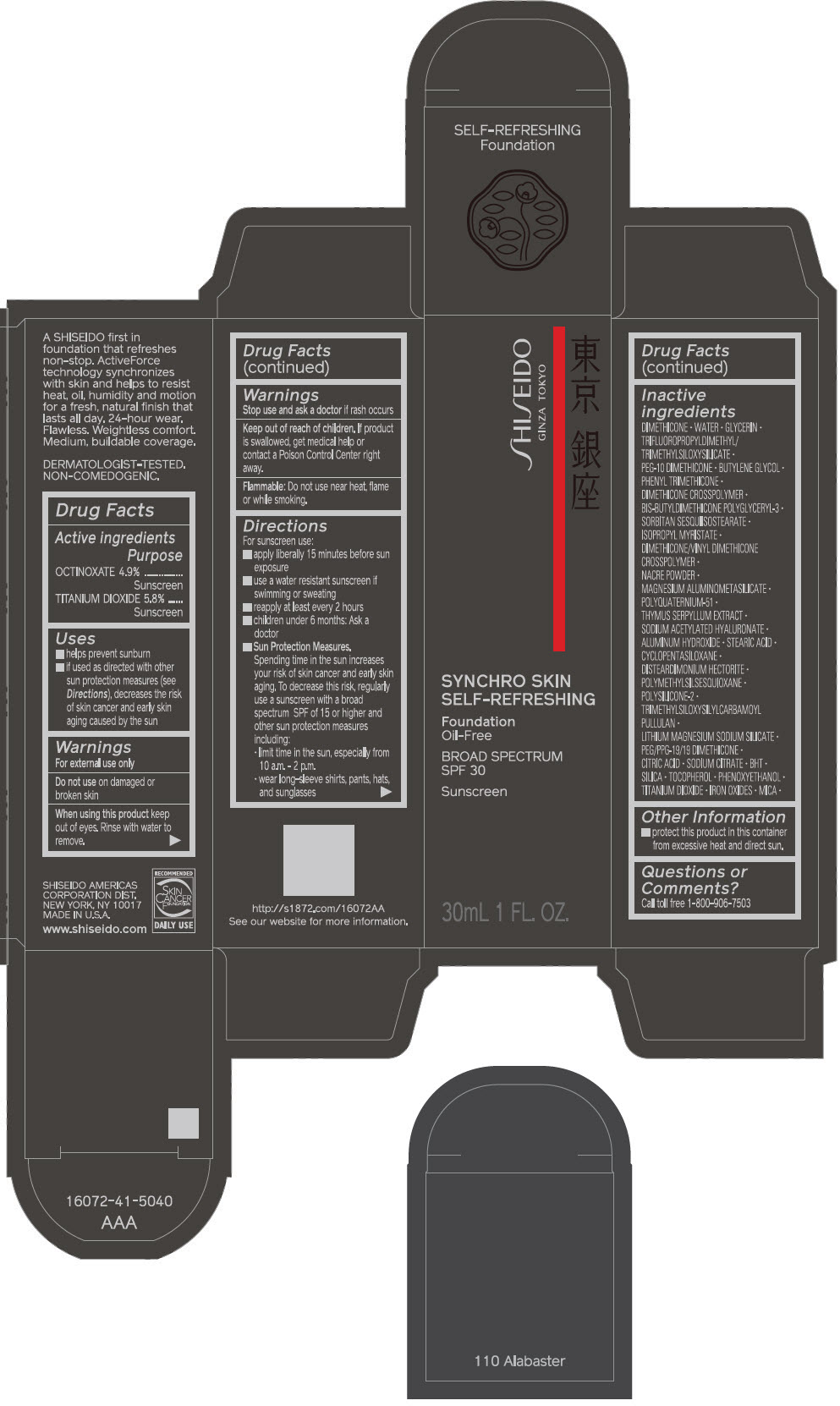
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 110 Alabaster
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 120 Ivory
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
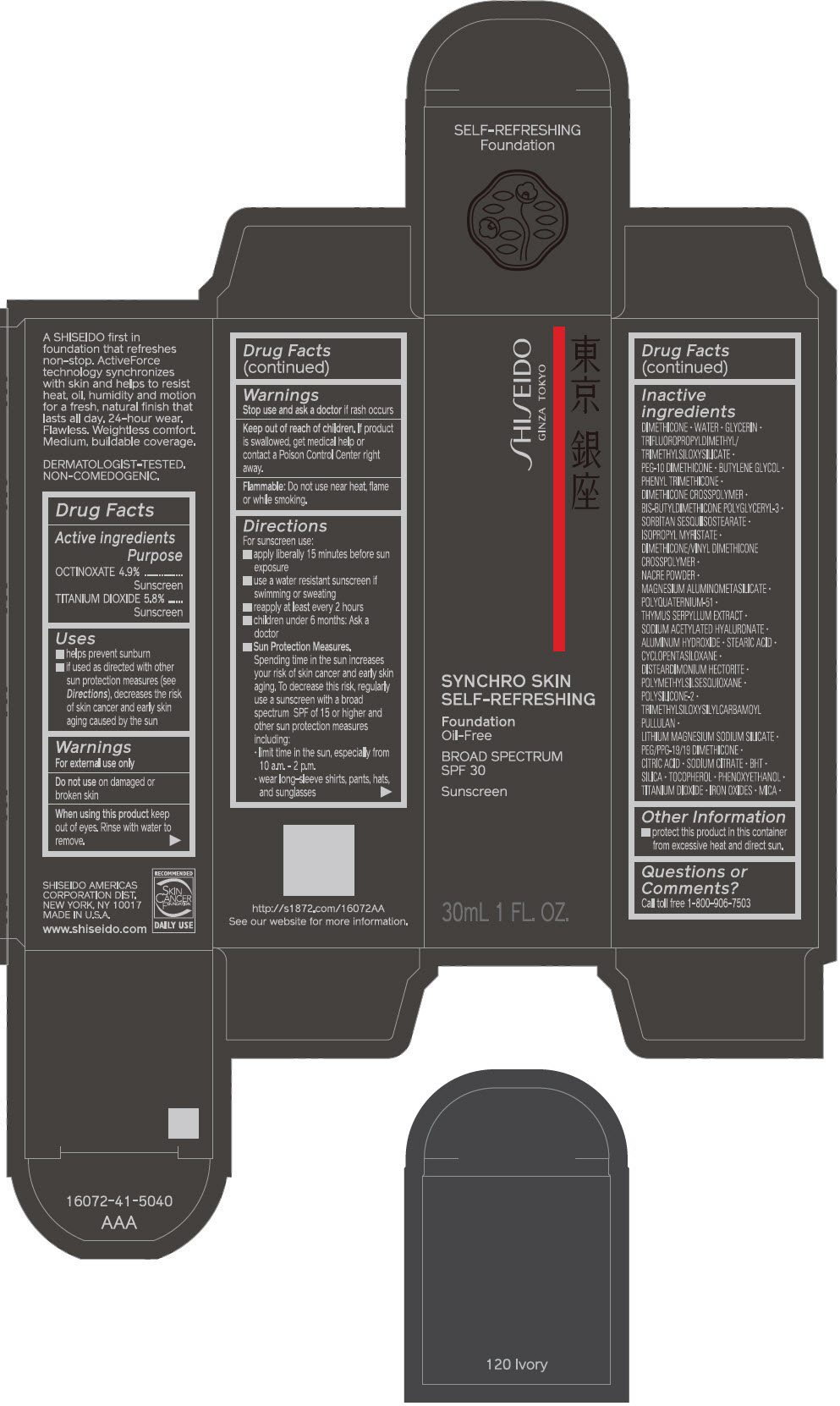
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 130 Opal
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
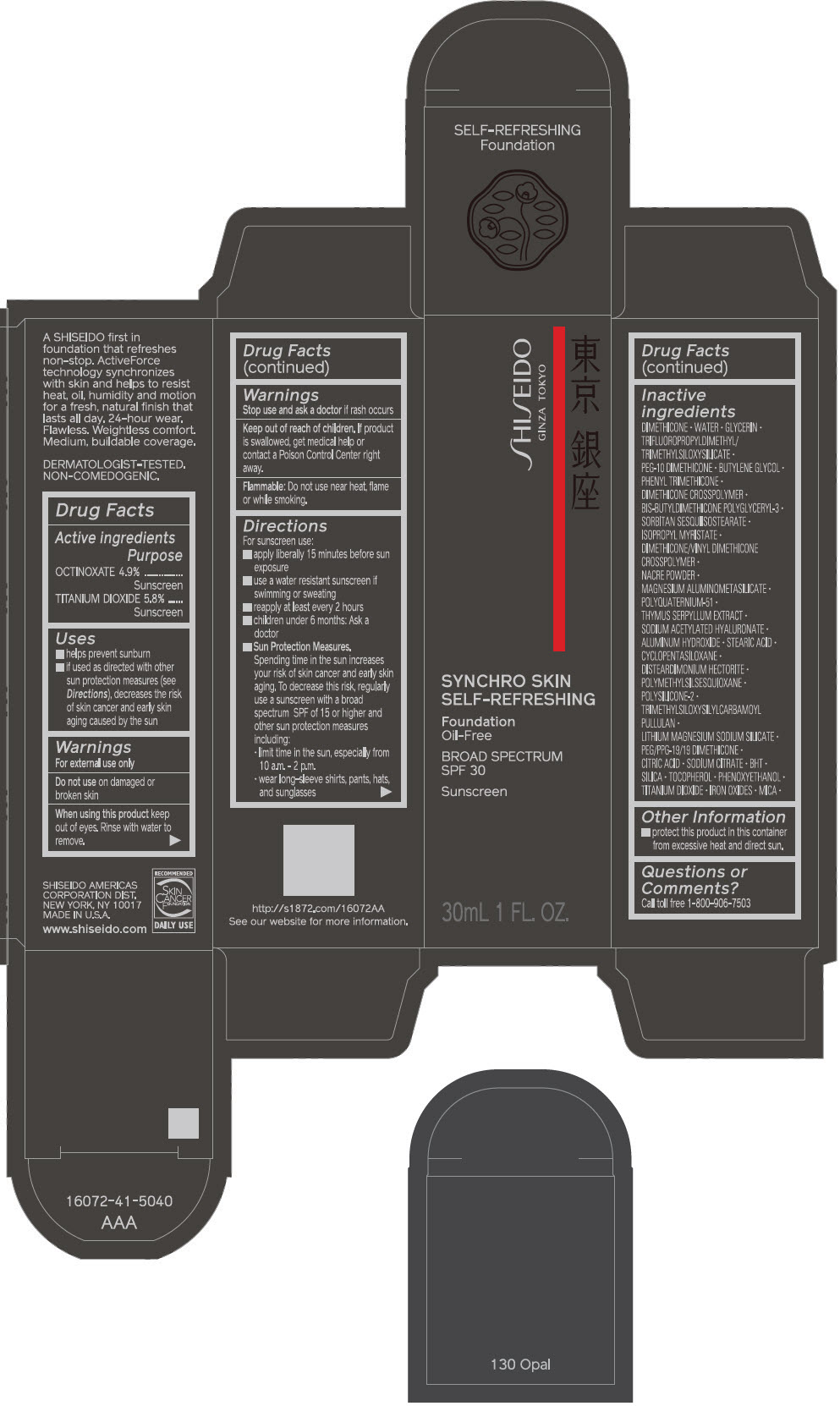
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 140 Porcelain
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
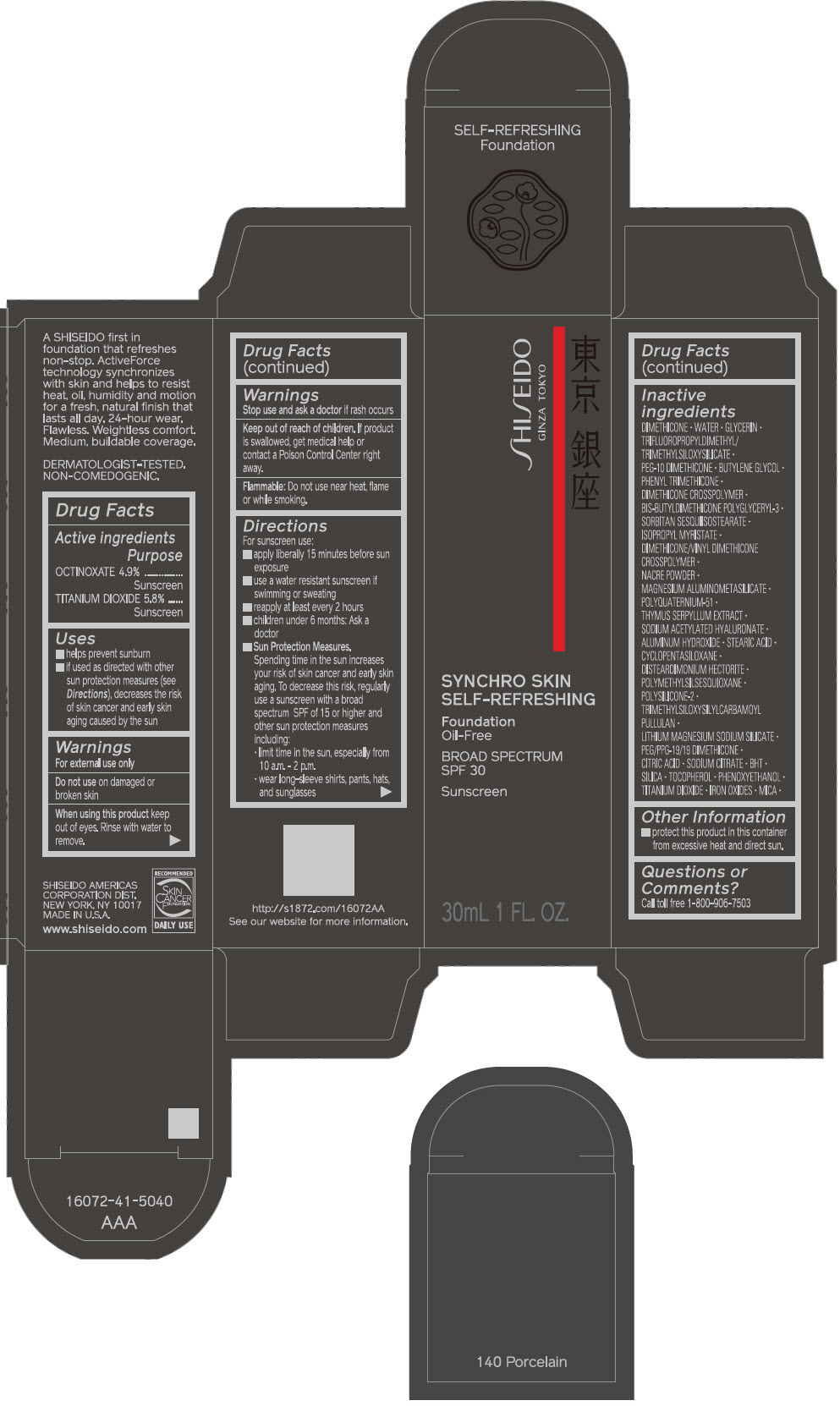
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 150 Lace
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
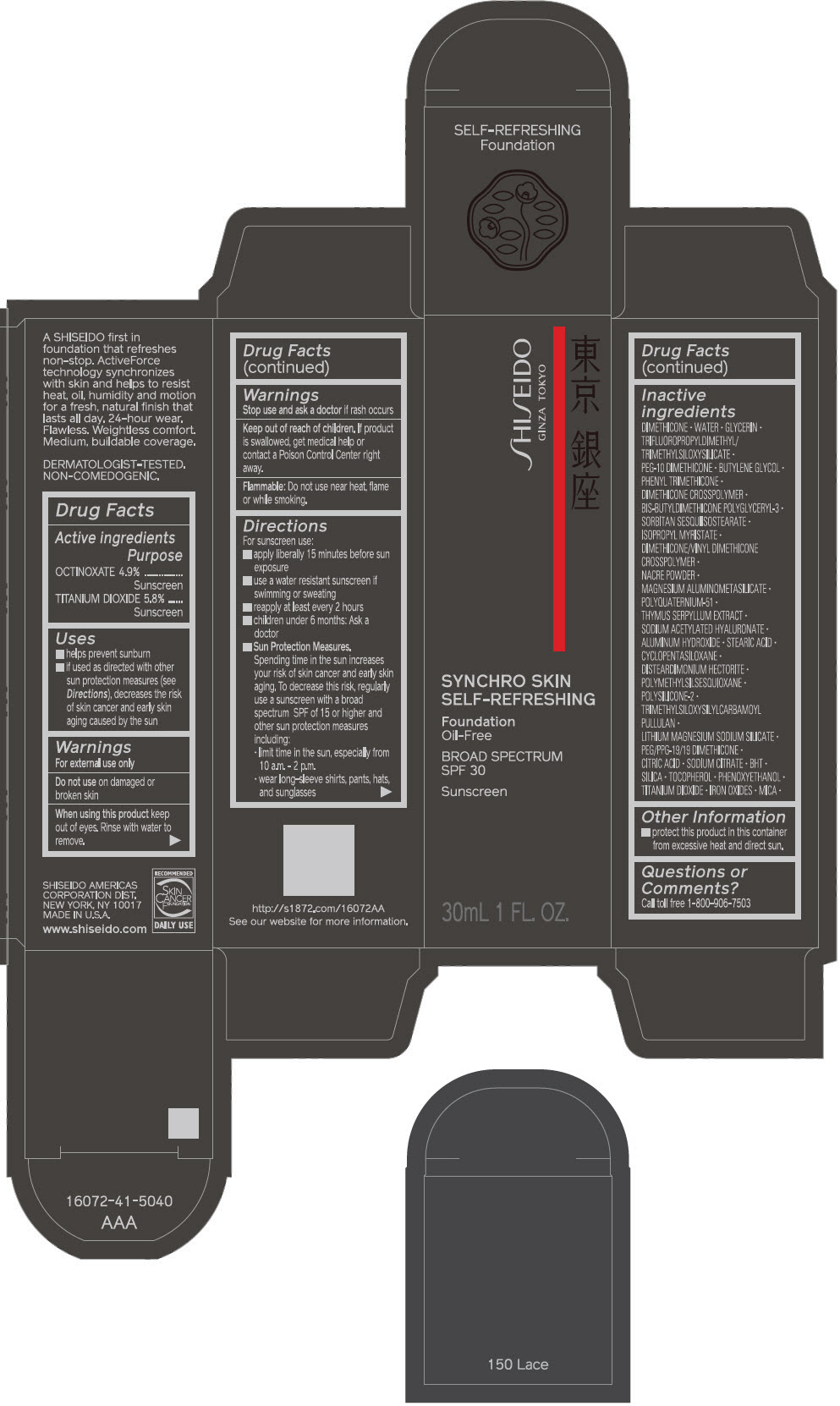
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 160 Shell
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
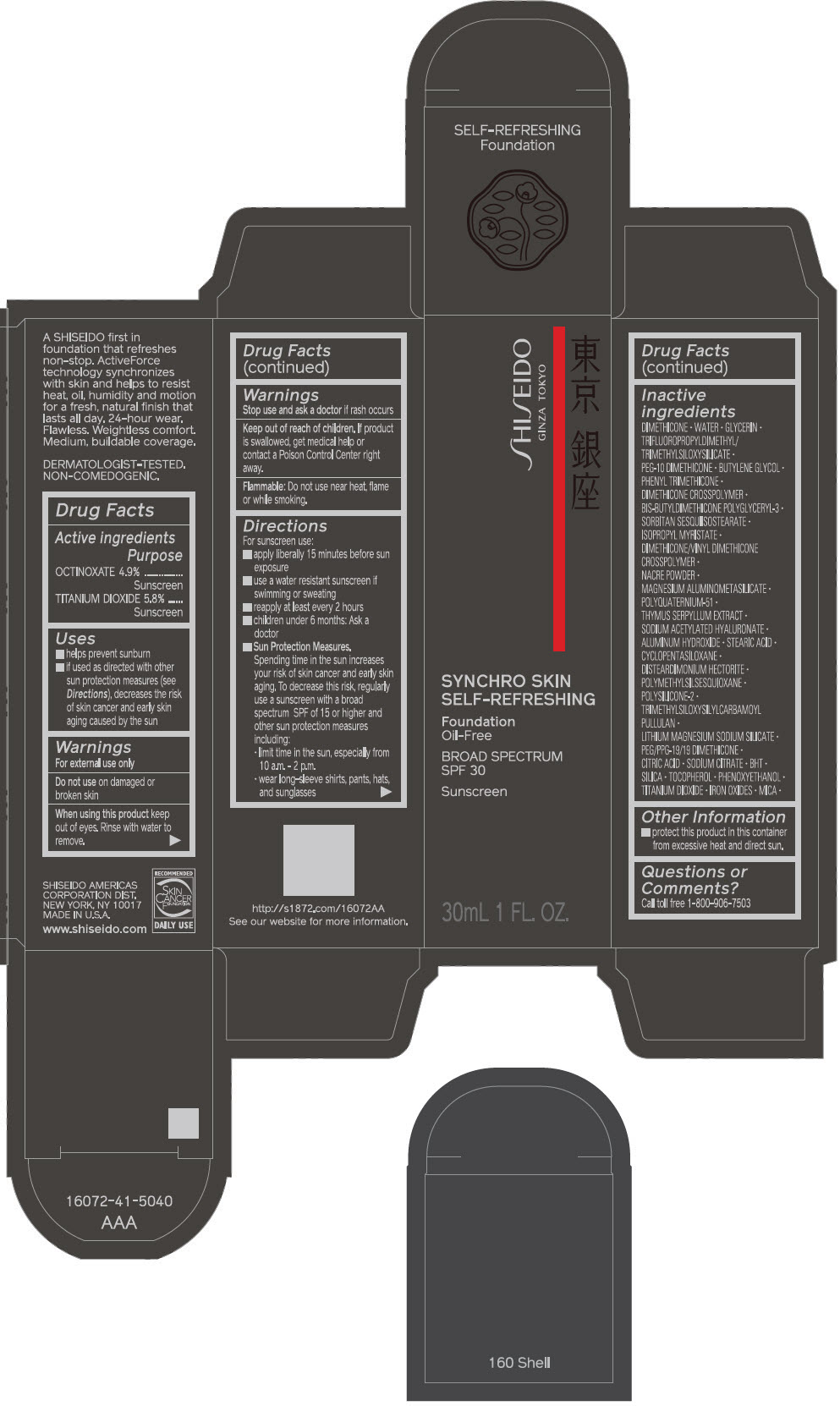
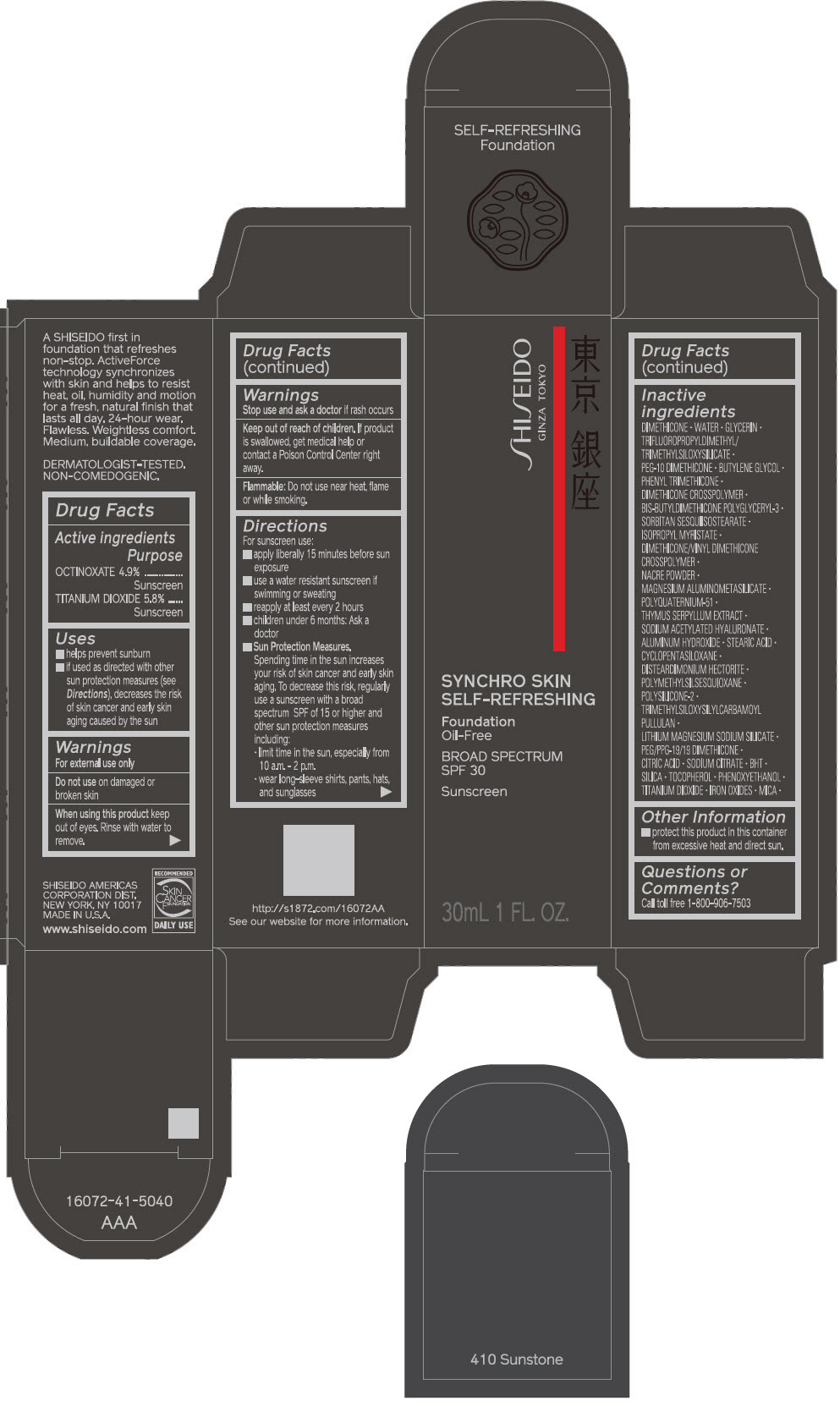
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 210 Birch
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
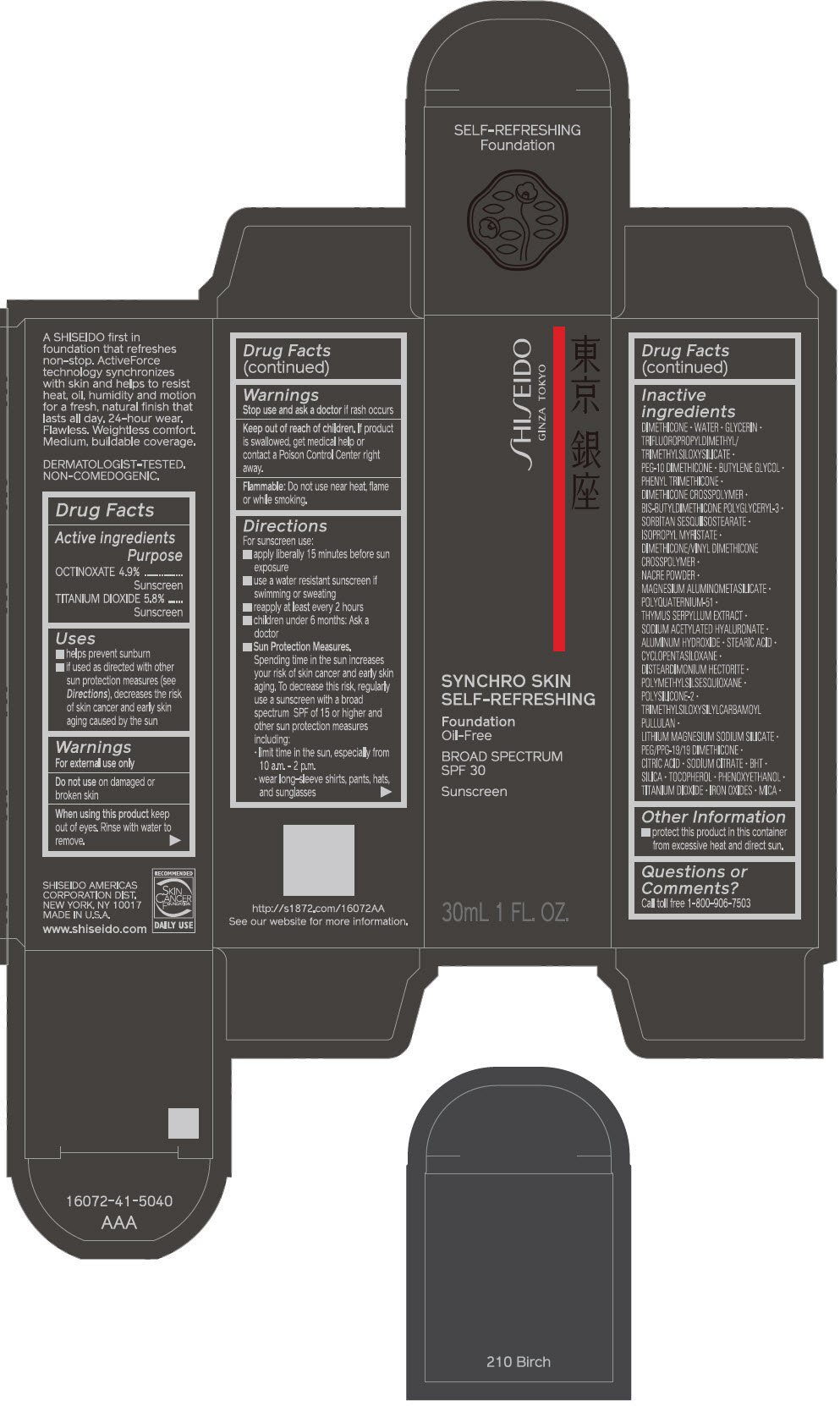
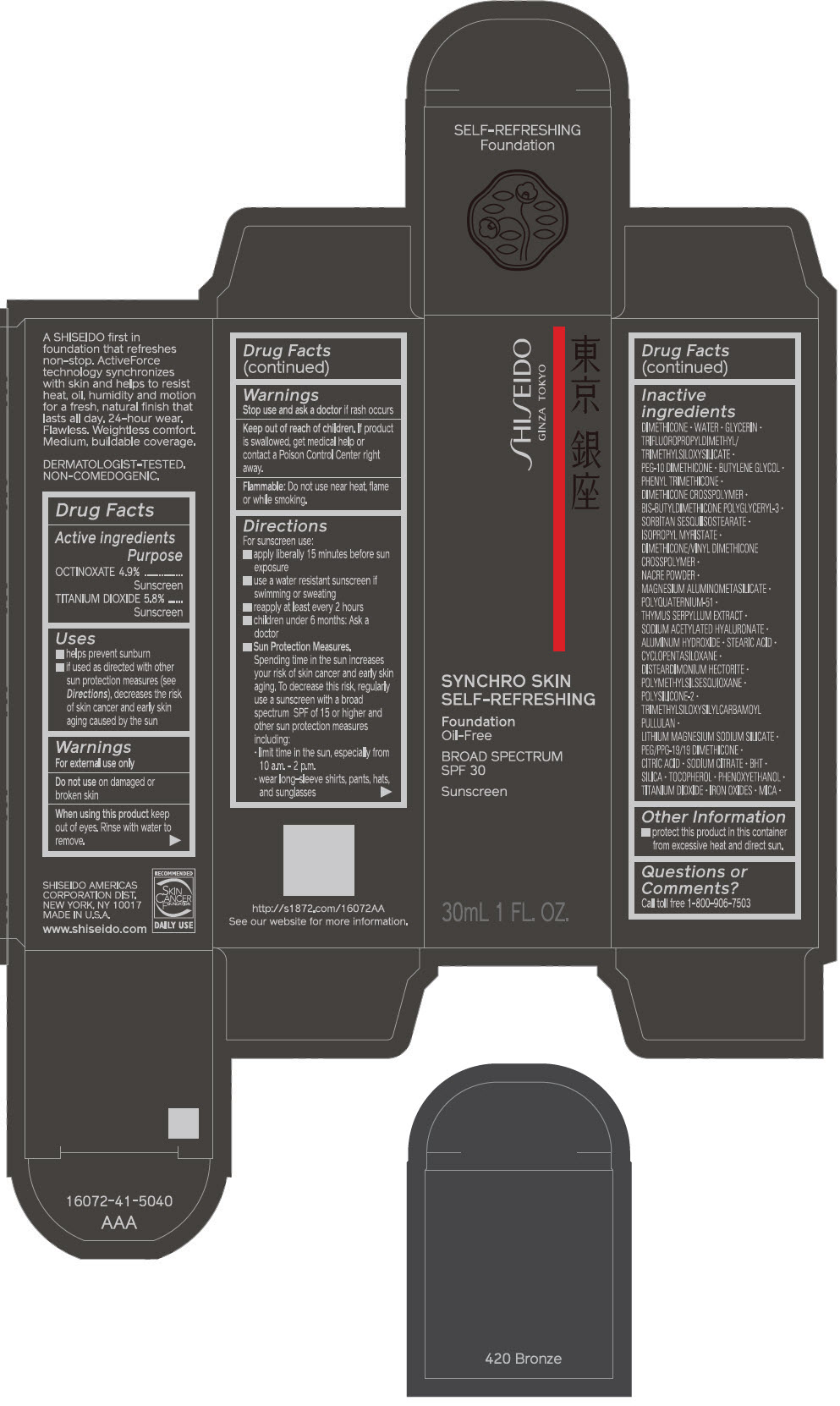
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 220 Linen
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
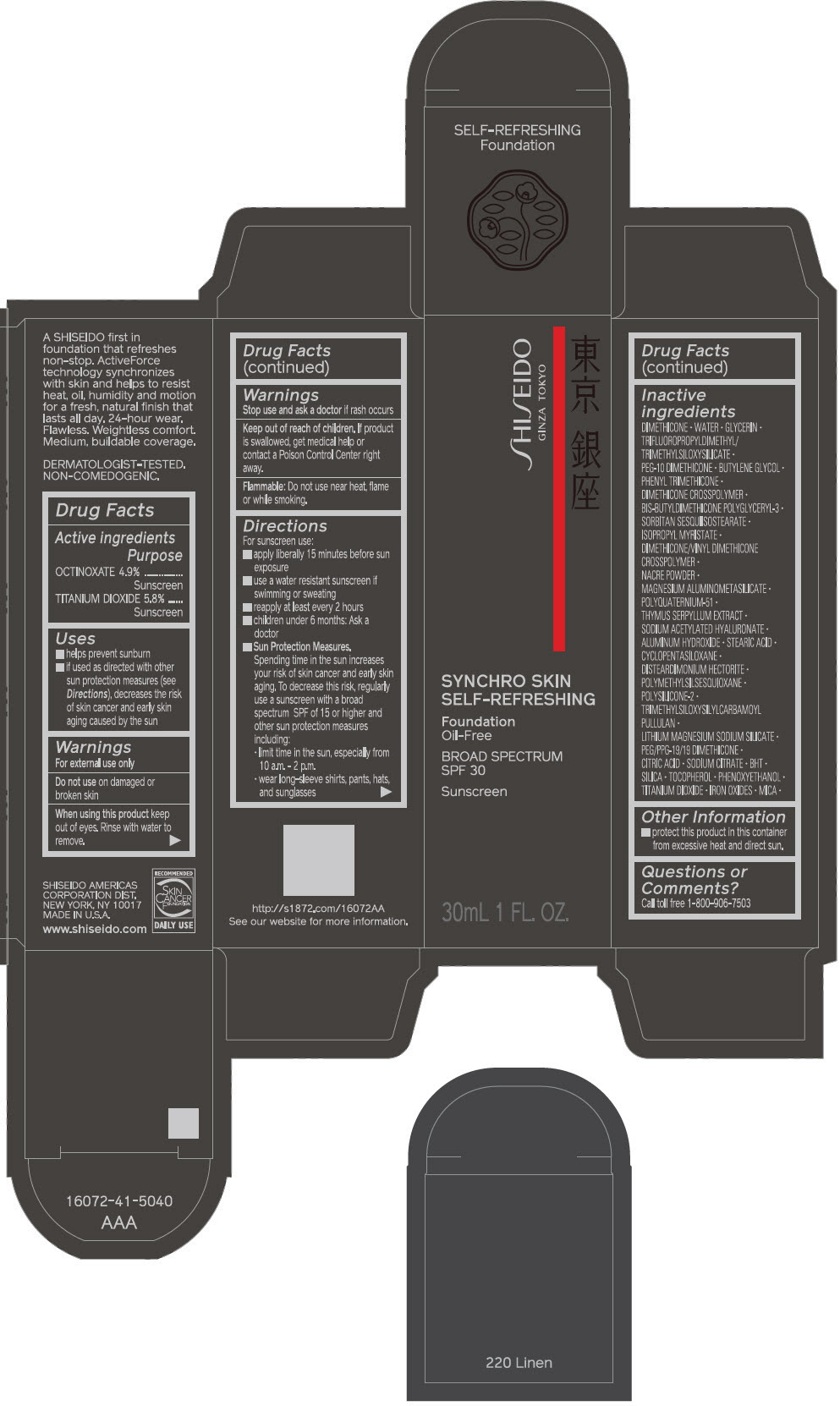
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 230 Alder
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
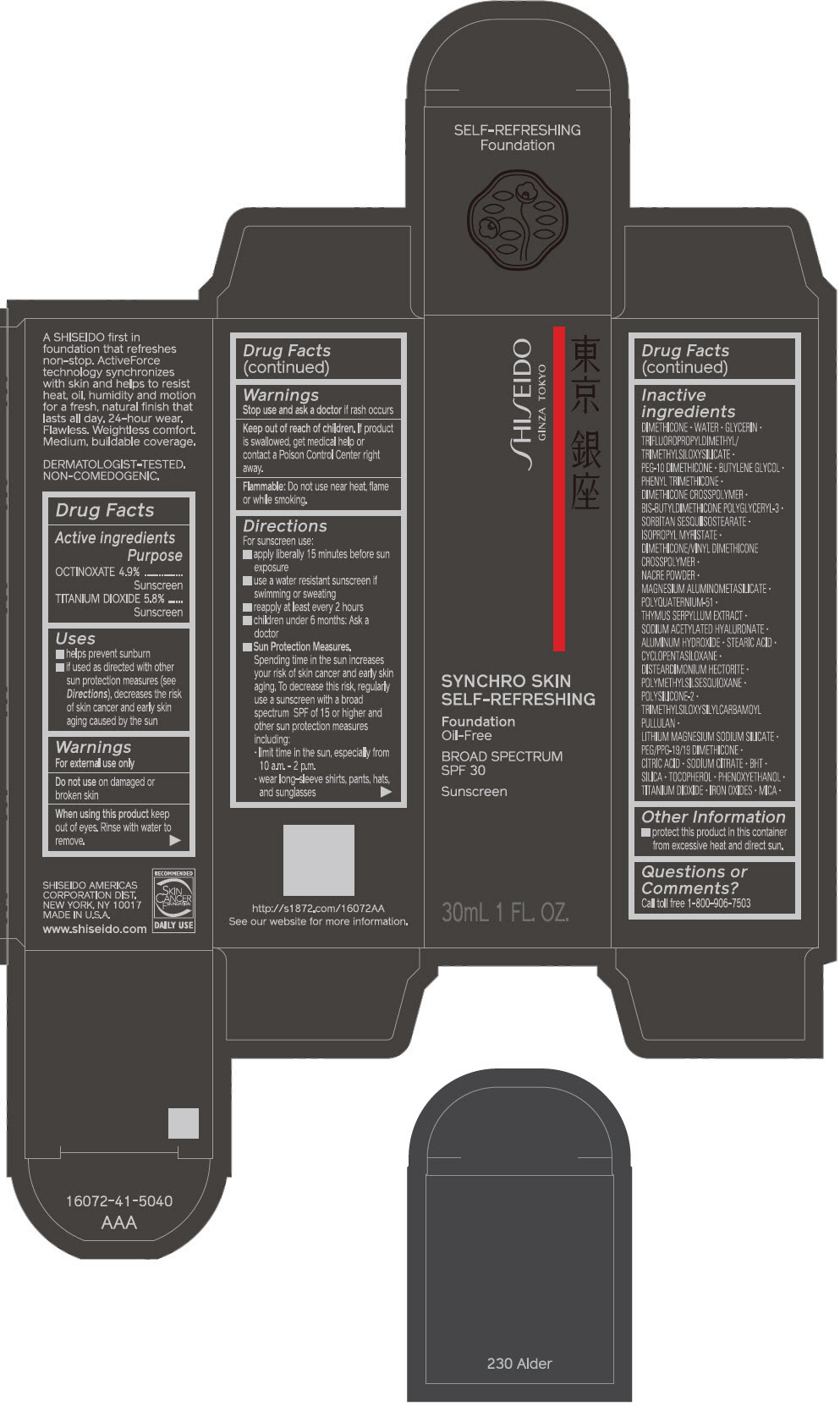
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 240 Quartz
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
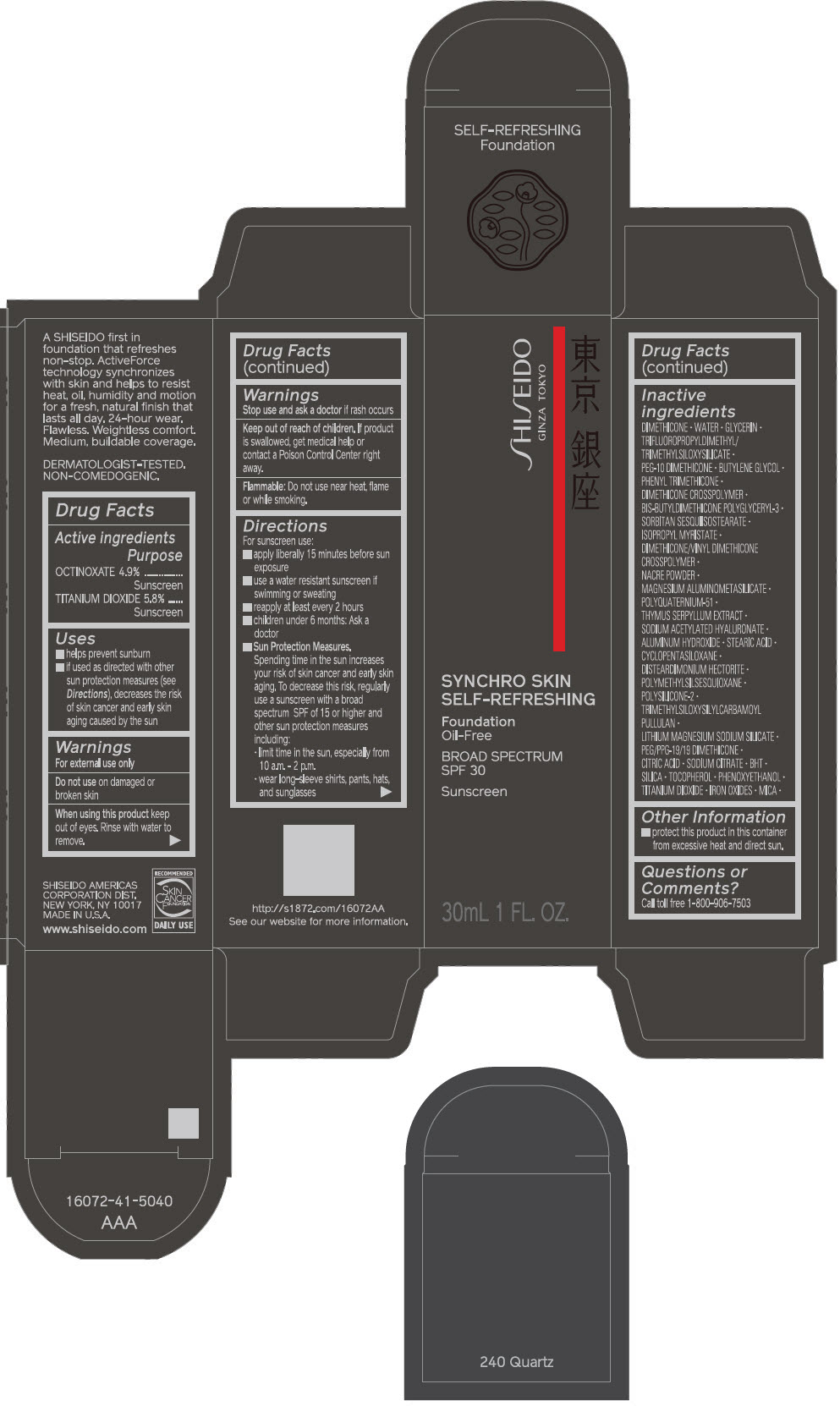
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 250 Sand
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
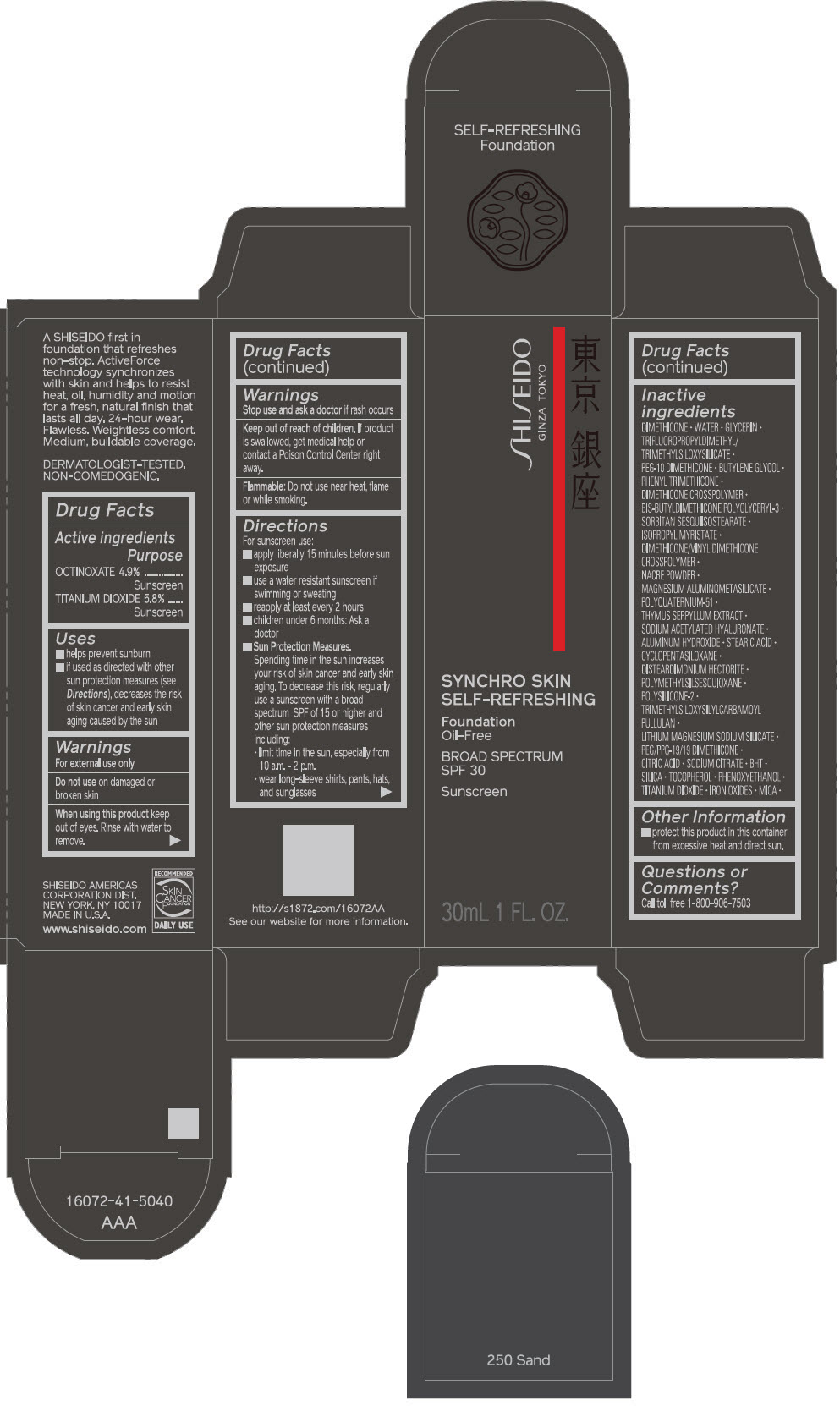
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 260 Cashmere
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
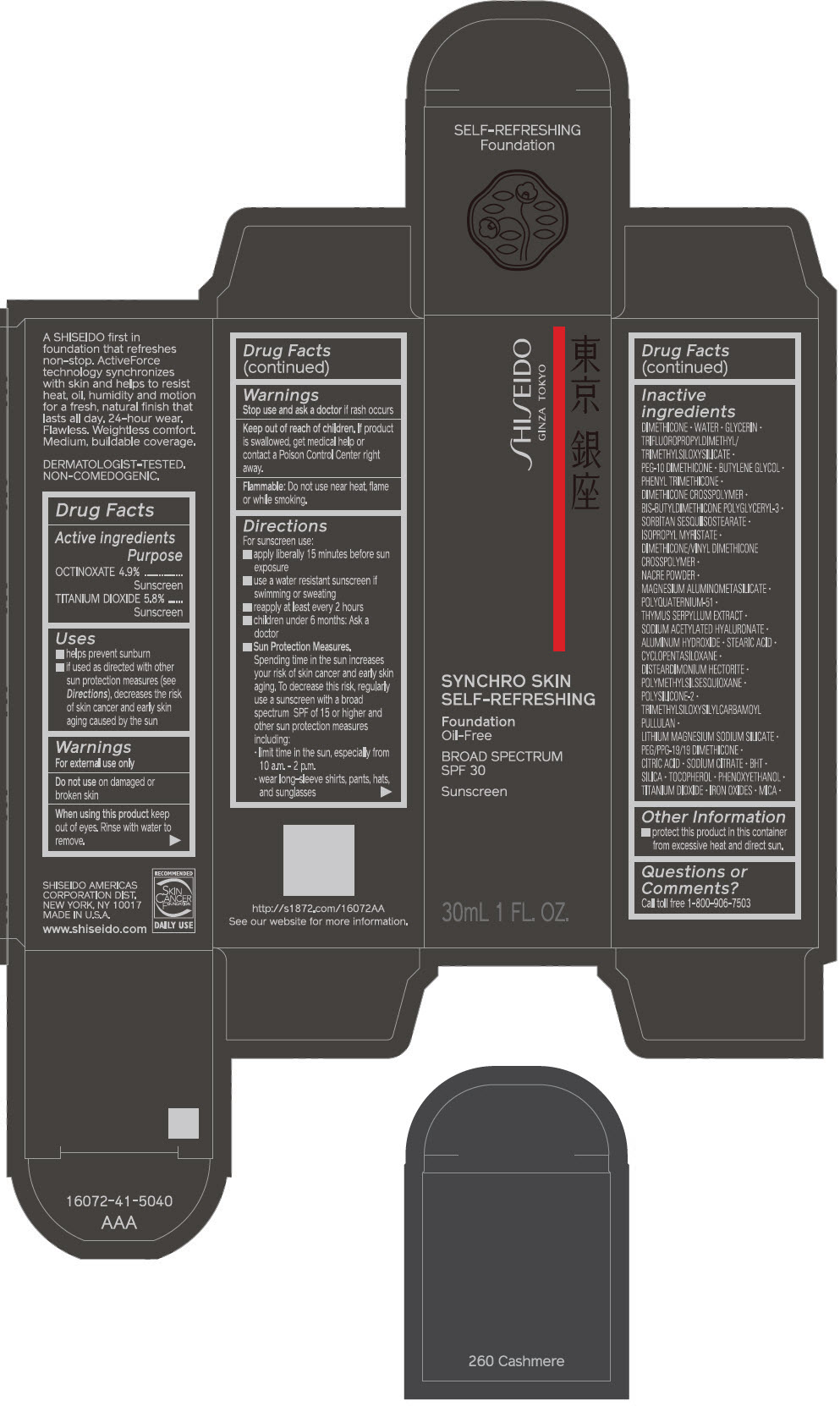
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 310 Silk
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
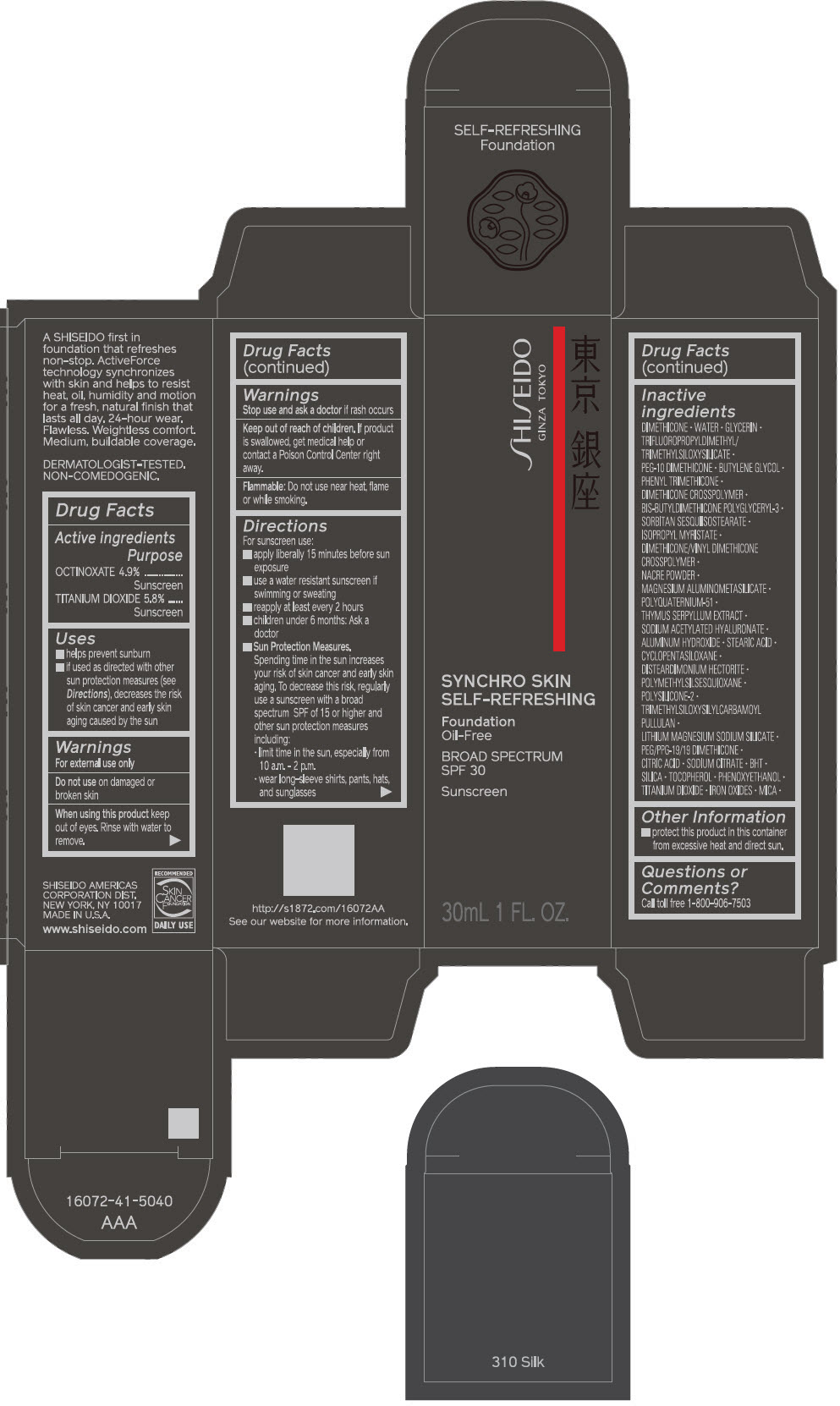
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 320 Pine
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
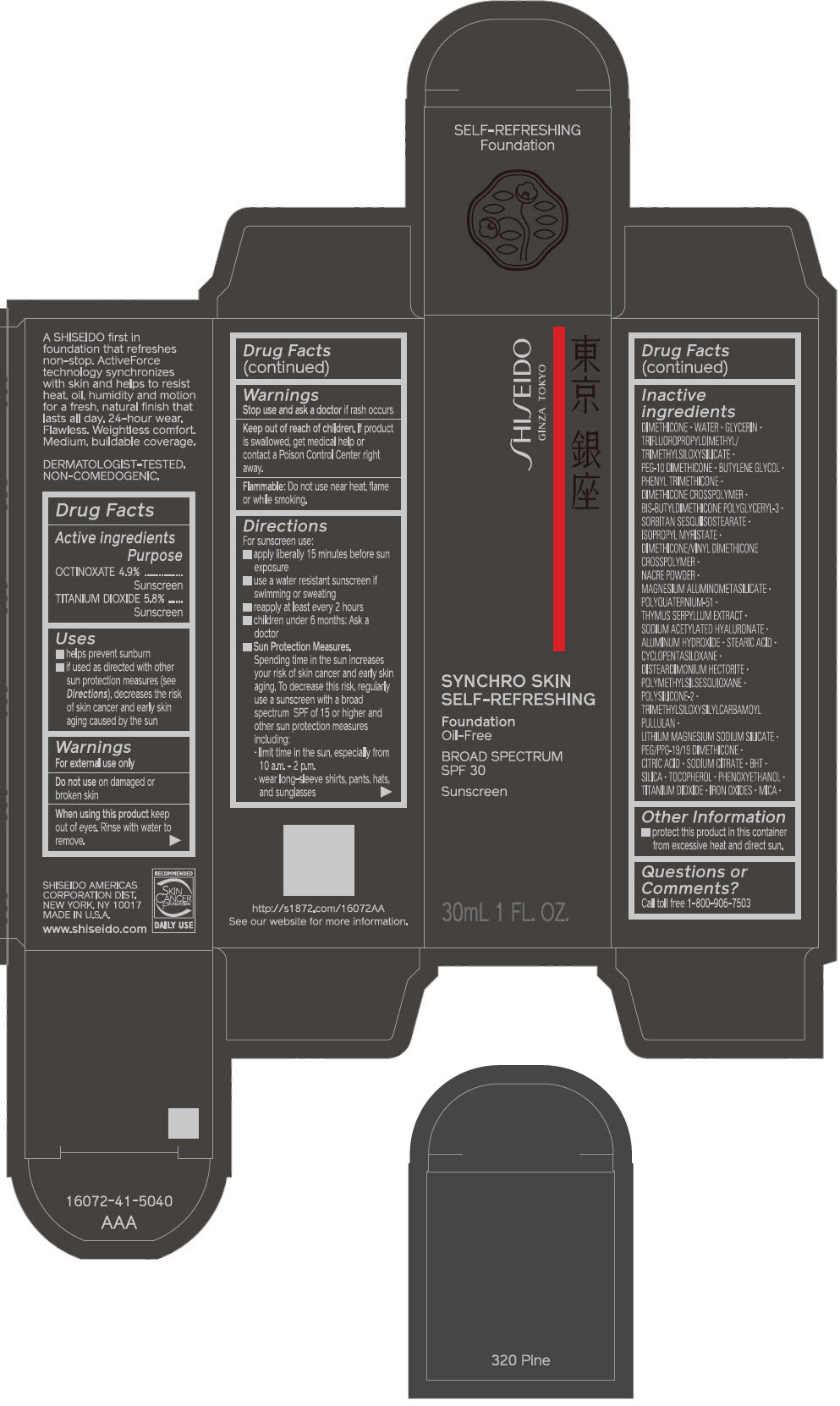
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 330 Bamboo
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
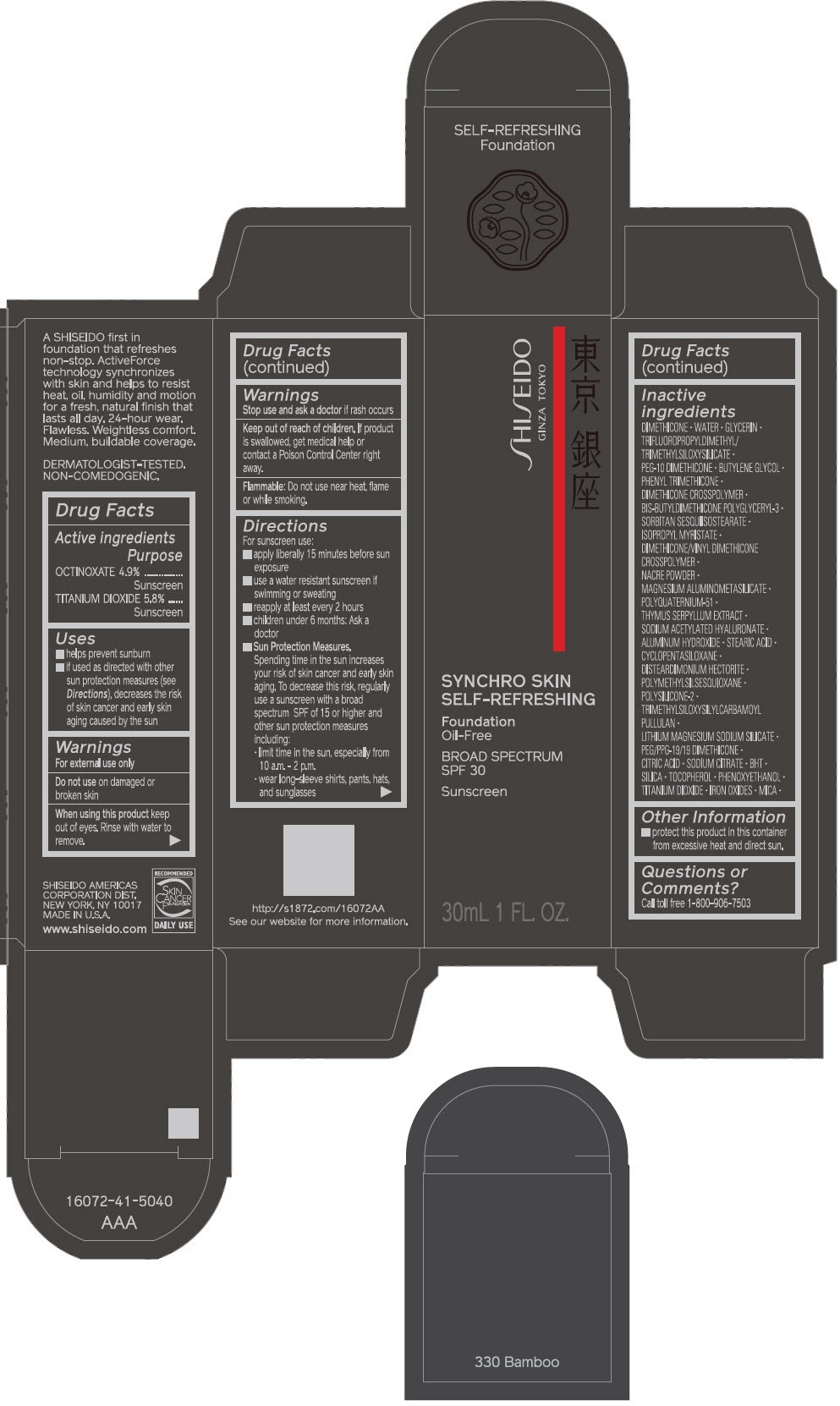
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 340 Oak
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
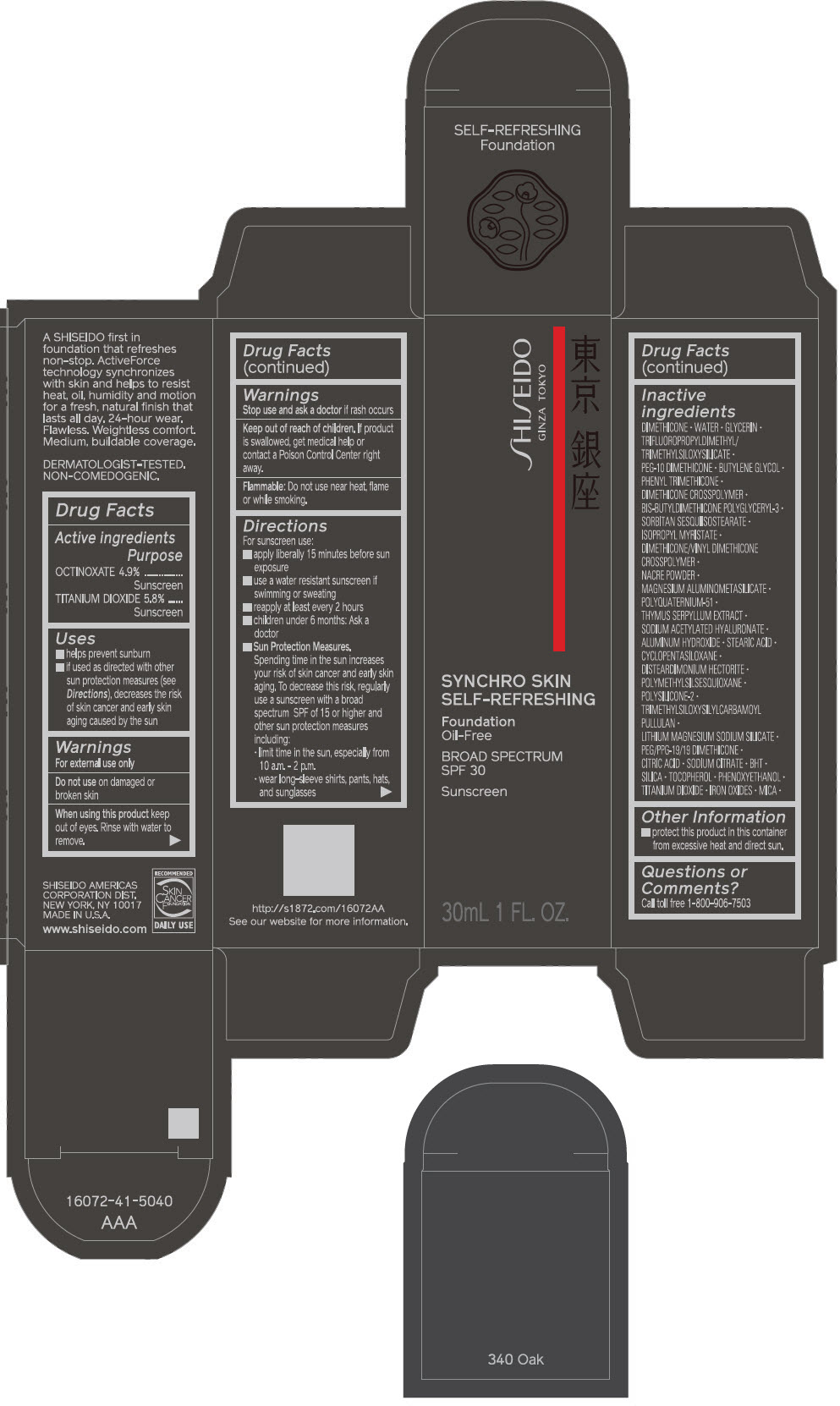
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 350 Maple
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.
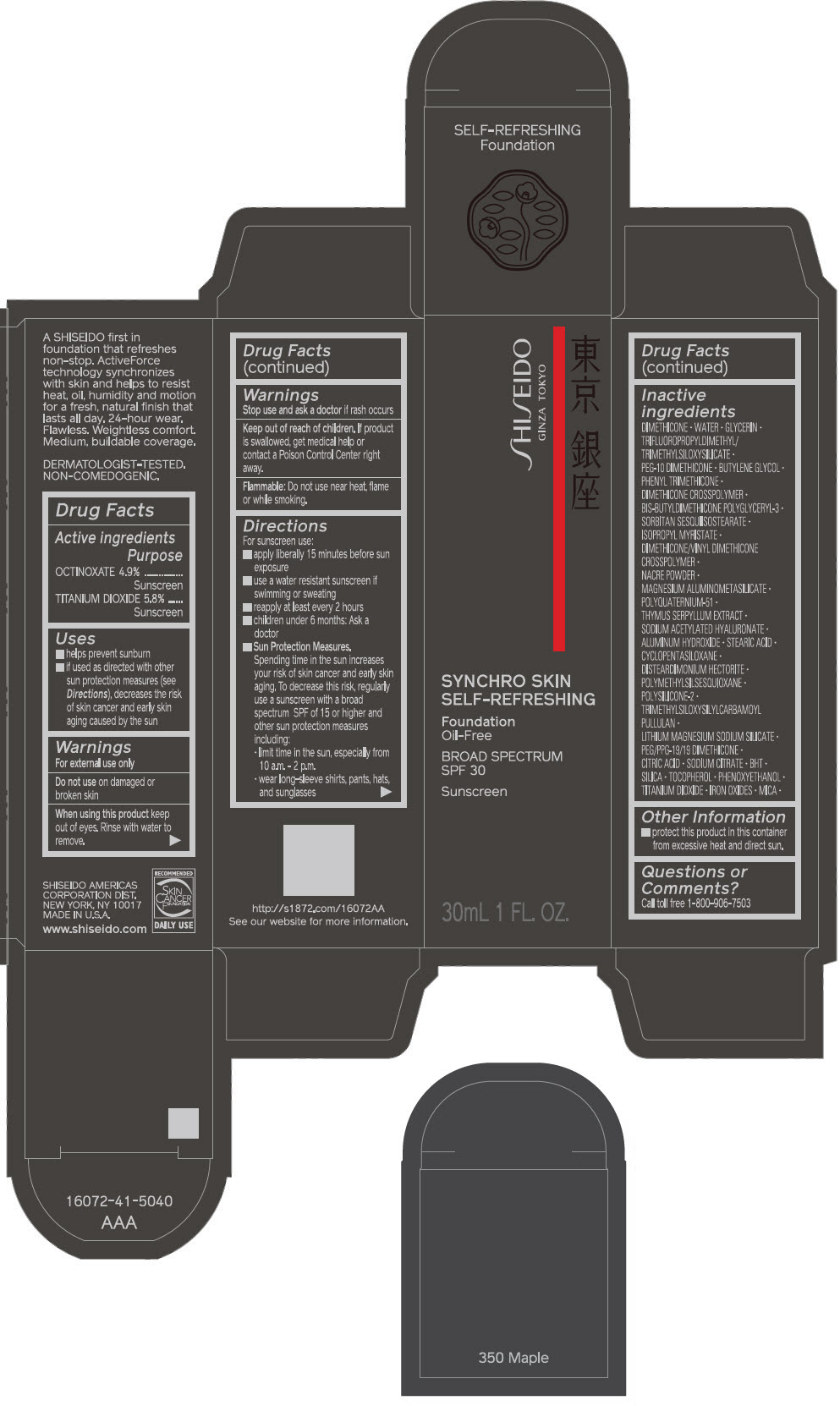
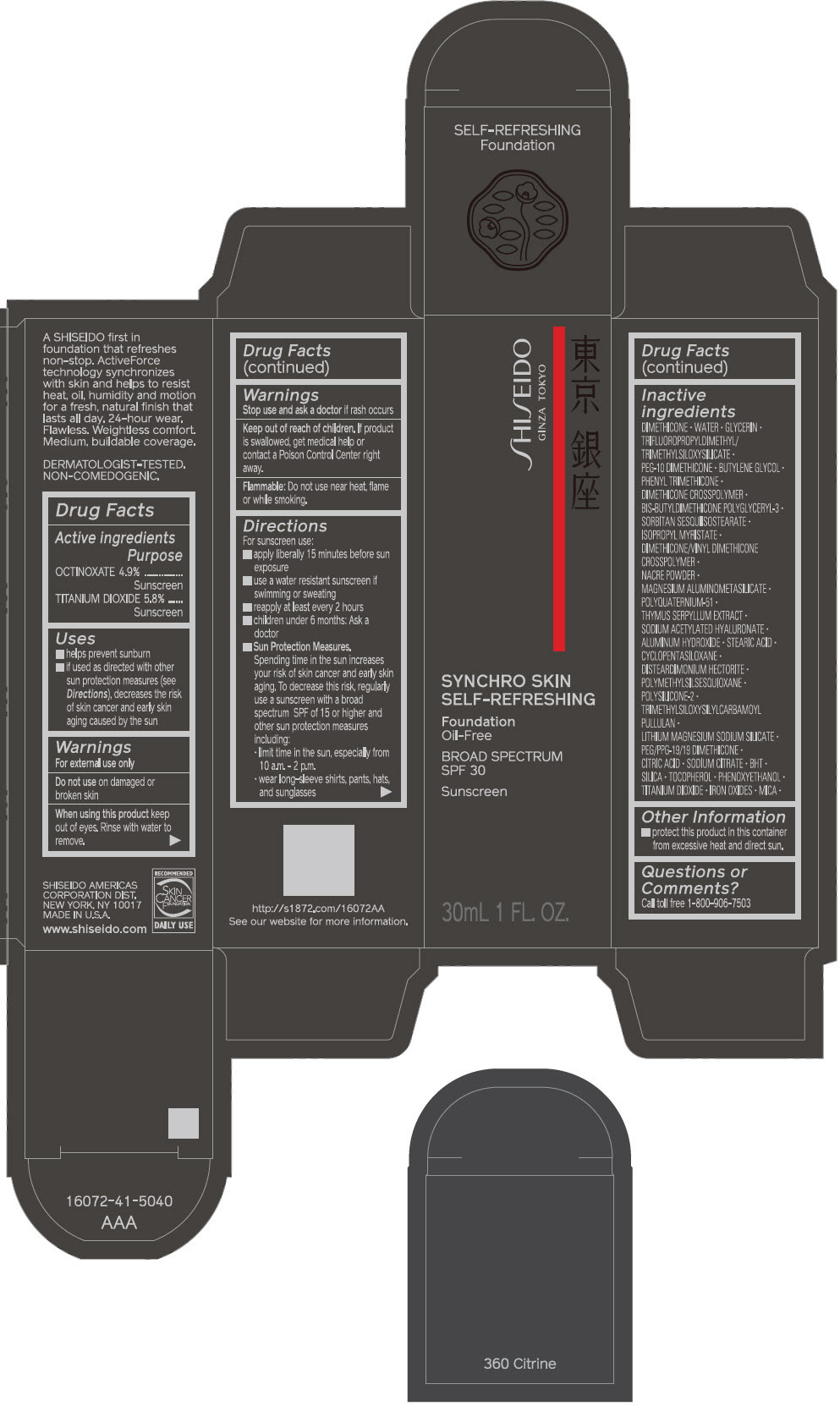
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 360 Citrine
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
SELF-REFRESHING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL 1 FL. OZ.