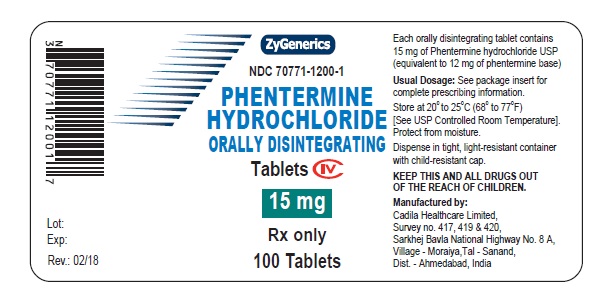
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1200-1 in bottle of 100 Tablets
Phentermine Hydrochloride Orally Disintegrating Tablets, 15 mg
Rx only
100 Tablets
NDC 70771-1201-1 in bottle of 100 Tablets
Phentermine Hydrochloride Orally Disintegrating Tablets, 30 mg
Rx only
100 Tablets

NDC 70771-1202-1 in bottle of 100 Tablets
Phentermine Hydrochloride Orally Disintegrating Tablets, 37.5 mg
Rx only
100 Tablets
