BOXED WARNING SECTION
WARNING: Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets, USP is intended for oral use only. Severe, potentially lethal, reactions may result from misuse of Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets, USP by injection either alone or in combination with other substances. (See DRUG ABUSE AND DEPENDENCE section.)
DESCRIPTION SECTION
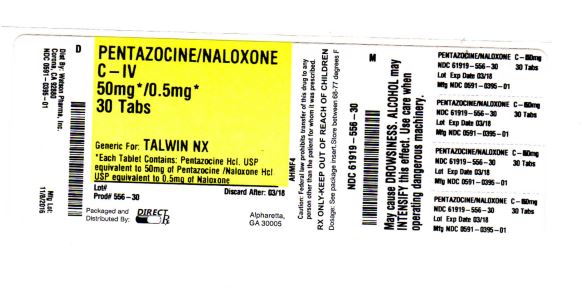
Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets contains pentazocine hydrochloride, USP, equivalent to 50 mg base and is a member of the benzazocine series (also known as the benzomorphan series), and naloxone hydrochloride, USP, equivalent to 0.5 mg base.
Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets, USP is an analgesic for oral administration.
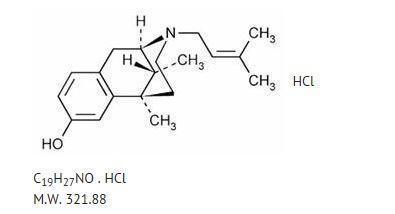
Chemically, pentazocine hydrochloride is (2R*,6R*,11R*)-1,2,3,4,5,6-Hexahydro-6,11dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol hydrochloride, a white, crystalline substance soluble in acidic aqueous solutions, and has the following structural formula:
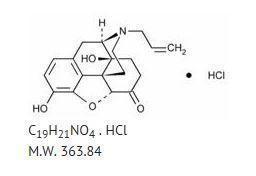
Chemically, naloxone hydrochloride is Morphinan-6-one,4,5-epoxy-3,14-dihydroxy-17-(2propenyl)-, hydrochloride, (5α)-. It is a slightly off-white powder, and is soluble in water and dilute acids, and has the following structural formula:
CLINICAL PHARMACOLOGY SECTION
Pentazocine is a Schedule IV opioid analgesic which when administered orally in a 50 mg dose appears equivalent in analgesic effect to 60 mg of codeine.
Pentazocine weakly antagonizes the analgesic effects of morphine and meperidine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity.
Onset of significant analgesia usually occurs between 15 and 30 minutes after oral administration, and duration of action is usually three hours or longer.
Pentazocine is well absorbed from the gastrointestinal tract. Concentrations in plasma coincide closely with the onset, duration, and intensity of analgesia. The time to mean peak concentration in 24 normal volunteers was 1.7 hours (range 0.5 to 4 hours) after oral administration and the mean plasma elimination half-life was 3.6 hours (range 1.5 to 10 hours).
Pentazocine is metabolized in the liver and excreted primarily in the urine. The products of the oxidation of the terminal methyl groups and glucuronide conjugates are excreted by the kidney. Elimination of approximately 60% of the total dose occurs within 24 hours. Pentazocine passes into the fetal circulation.
Naloxone when administered orally at 0.5 mg has no pharmacologic activity. Naloxone hydrochloride administered parenterally at the same dose is an antagonist to pentazocine and a pure antagonist to narcotic analgesics.
Pentazocine hydrochloride and naloxone hydrochloride is a potent analgesic when administered orally. However, the presence of naloxone in Pentazocine hydrochloride and naloxone hydrochloride is intended to prevent the effect of pentazocine if the product is misused by injection.
Studies in animals indicate that the presence of naloxone does not affect pentazocine analgesia when the combination is given orally. If the combination is given by injection the action of pentazocine is neutralized.
INDICATIONS & USAGE SECTION
Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets, USP is indicated for the relief of moderate to severe pain.
Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets, USP is indicated for oral use only.
CONTRAINDICATIONS SECTION
Pentazocine hydrochloride and naloxone hydrochloride tablets is contraindicated in patients who are hypersensitive to either pentazocine or naloxone.
DRUG ABUSE AND DEPENDENCE SECTION
Pentazocine can cause a physical and psychological dependence. (See DRUG ABUSE AND DEPENDENCE.)
Use In Head Injury and Increased Intracranial Pressure
In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the possible respiratory depressant effects of pentazocine and its potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly increased. Furthermore, pentazocine can produce effects on pupillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries. In such patients, pentazocine must be used with extreme caution and only if its use is deemed essential.
Interactions with Alcohol and Drugs of Abuse
Pentazocine may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression because respiratory depression, hypotension, profound sedation, coma or death may result.
Patients Receiving Narcotics
Pentazocine is a mild narcotic antagonist. Some patients previously given narcotics, including methadone for the daily treatment of narcotic dependence, have experienced withdrawal symptoms after receiving pentazocine.
Respiratory Depression
Respiratory depression occurs more frequently in elderly or debilitated patients and in those suffering from conditions accompanied by hypoxia, hypercapnia, or upper airway obstruction, in whom even moderate therapeutic doses may significantly decrease pulmonary ventilation. Use pentazocine hydrochloride and naloxone hydrochloride with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale and in patients having a substantially decreased respiratory reserve (e.g., severe kyphoscoliosis), hypoxia, hypercapnia, or pre-existing respiratory depression. Alternative non-opioid analgesics should be considered, and pentazocine hydrochloride and naloxone hydrochloride should be employed only under careful medical supervision at the lowest effective dose in such patients.
Acute CNS Manifestations
Patients receiving therapeutic doses of pentazocine hydrochloride and naloxone hydrochloride have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be very closely observed and vital signs checked. If the drug is reinstituted, it
PRECAUTIONS SECTION
- Drug Abuse and Dependence
Pentazocine hydrochloride and naloxone hydrochloride tablets are Schedule IV controlled substance.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of a drug for non-medical purposes, often in combination with other psychoactive substances. Addiction is a disease of repeated drug abuse. Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. Addiction is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by misuse of the drug for non-medical purposes, and often in combination with other psychoactive substances.
There have been some reports of dependence and of withdrawal symptoms with pentazocine hydrochloride and naloxone hydrochloride. Patients with a history of drug dependence should be under close supervision while receiving Pentazocine hydrochloride and naloxone hydrochloride. There have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine hydrochloride and naloxone hydrochloride during pregnancy.
There have been reports of development of addiction and physical dependence in patients receiving parenteral pentazocine. People with a history of drug abuse or alcohol abuse may have a higher chance of becoming addicted to opioid medicines.
Abrupt dose cessation or rapid dose reduction following the extended use of parenteral pentazocine has resulted in withdrawal symptoms such as abdominal cramps, nausea, vomiting, elevated temperature, chills, rhinorrhea, restlessness, anxiety, or lacrimation. In general opioid therapy should not be abruptly discontinued. When the patient no longer requires treatment with pentazocine hydrochloride and naloxone hydrochloride, the drug should be tapered gradually to prevent signs and symptoms of withdrawal in patients who have been receiving opioids for an extended period of time and might have become physically dependent.
In prescribing pentazocine hydrochloride and naloxone hydrochloride for chronic use, the physician should take under consideration that proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to identify and decrease misuse and abuse of opioid drugs.
The amount of naloxone present in pentazocine hydrochloride and naloxone hydrochloride (0.5 mg per tablet) has no action when taken orally and will not interfere with the pharmacologic action of pentazocine. However, this amount of naloxone given by injection has profound antagonistic action to narcotic analgesics.
Severe, even lethal, consequences may result from misuse of tablets by injection either alone or in combination with other substances, such as pulmonary emboli, vascular occlusion, ulceration and abscesses, and withdrawal symptoms in narcotic dependent individuals.
CNS Effect
Caution should be used when pentazocine hydrochloride and naloxone hydrochloride is administered to patients prone to seizures; seizures have occurred in a few such patients in association with the use of pentazocine though no cause and effect relationship has been established.
Porphyria
Particular caution should be exercised in administering pentazocine to patients with porphyria since it may provoke an acute attack in susceptible individuals.
Cardiovascular Disease
Pentazocine can elevate blood pressure, possibly through the release of endogenous catecholamines. Particular caution should be exercised in conditions where alterations in vascular resistance and blood pressure might be particularly undesirable, such as in the acute phase of myocardial infarction.
Pentazocine hydrochloride and naloxone hydrochloride should be used with caution in patients with myocardial infarction who have nausea or vomiting.
Impaired Renal or Hepatic Function
Decreased metabolism of pentazocine by the liver in extensive liver disease may predispose to accentuation of side effects. Although laboratory tests have not indicated that pentazocine causes or increases renal or hepatic impairment, the drug should be administered with caution to patients with such impairment.
Other
Caution should also be observed when administering pentazocine hydrochloride and naloxone hydrochloride in patients with hypothyroidism, adrenocortical insufficiency, prostate hypertrophy, inflammatory or obstructive bowel disease, acute abdominal syndromes of unknown etiology, cholecystitis, pancreatitis, or acute alcohol intoxication and delirium tremens.
Biliary Surgery
Narcotic drug products are generally considered to elevate biliary tract pressure for varying periods following their administration. Some evidence suggests that pentazocine may differ from other marketed narcotics in this respect (i.e., it causes little or no elevation in biliary tract pressures). The clinical significance of these findings, however, is not yet known.
Information for Patients- Patients receiving pentazocine hydrochloride and naloxone hydrochloride should be given the following instructions by the physician:
- Patients should be advised that pentazocine hydrochloride and naloxone hydrochloride is a narcotic pain reliever, and should be taken only as directed.
- The dose of pentazocine hydrochloride and naloxone hydrochloride should not be adjusted without consulting with a physician or other healthcare professional.
- Patients should be advised that pentazocine hydrochloride and naloxone hydrochloride may cause drowsiness, dizziness, or lightheadedness and may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating machinery). Patients started on pentazocine hydrochloride and naloxone hydrochloride or patients whose dose has been adjusted should refrain from any potentially dangerous activity until it is established that they are not adversely affected.
- Pentazocine hydrochloride and naloxone hydrochloride will add to the effect of alcohol and other CNS depressants (such as antihistamines, sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and monoamine oxidase [MAO]inhibitors).
- Patients should not combine pentazocine hydrochloride and naloxone hydrochloride with alcohol or other central nervous system depressants (sleep aids, tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death.
- Women of childbearing potential who become or are planning to become pregnant should consult a physician prior to initiating or continuing therapy with pentazocine hydrochloride and naloxone hydrochloride.
- Safe use in pregnancy has not been established. Prolonged use of opioid analgesics during pregnancy may cause neonatal physical dependence, and neonatal withdrawal may occur.
- If patients have been receiving treatment with pentazocine hydrochloride and naloxone hydrochloride for more than a few weeks and cessation of therapy is indicated, they should be counseled on the importance of safely tapering the dose and that abruptly discontinuing the medication could precipitate withdrawal symptoms. The physician should provide a dose schedule to accomplish a gradual discontinuation of the medication.
- Patients should be advised that pentazocine hydrochloride and naloxone hydrochloride is a potential drug of abuse. They should protect it from theft. It should never be given to anyone other than the individual for whom it was prescribed.
- Patients should be instructed to keep pentazocine hydrochloride and naloxone hydrochloride in a secure place out of the reach of children. When Pentazocine hydrochloride and naloxone hydrochloride is no longer needed, please consult your pharmacist for proper disposal instructions.
- As with other opioids, patients taking pentazocine hydrochloride and naloxone hydrochloride should be advised of the potential for severe constipation; appropriate laxatives and/or stool softeners as well as other appropriate treatments should be initiated from the onset of opioid therapy.
- Patients should be advised of the most common adverse events that may occur while taking pentazocine hydrochloride and naloxone hydrochloride: constipation, nausea, somnolence, lightheadedness, dizziness, sedation, vomiting, and sweating.
CNS Depressants
Other central nervous system (CNS) depressants including sedatives, hypnotics, general anesthetics, antiemetics, phenothiazines, or other tranquilizers or alcohol increases the risk of respiratory depression, hypotension, profound sedation, or coma. Use morphine sulfate with caution and in reduced dosages in patients taking these agents.
Opioid Agonist Analgesics
Pentazocine hydrochloride and naloxone hydrochloride can antagonize the effects of a pure opioid agonist analgesic and/or may precipitate withdrawal symptoms.
Monoamine Oxidase Inhibitors (MAOIs)
Concomitant use of monoamine oxidase inhibitors (MAOIs) with pentazocine hydrochloride and naloxone hydrochloride may cause CNS excitation and hypertension through their respective effects on catecholamines. Caution should therefore be observed in administering pentazocine hydrochloride and naloxone hydrochloride to patients who are currently receiving MAOIs or who have received them within the preceding 14 days.
Anticholinergics
Anticholinergics or other medications with anticholinergic activity when used concurrently with opioid analgesics may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
Tobacco
Smoking tobacco could enhance the metabolic clearance rate of pentazocine reducing the clinical effectiveness of a standard dose of pentazocine.
Carcinogenesis, Mutagenesis, Impairment of FertilityNo long-term studies in animals to test for carcinogenesis have been performed with the components of pentazocine hydrochloride and naloxone hydrochloride.
Studies to evaluate the mutagenic potential of the components of pentazocine hydrochloride and naloxone hydrochloride have not been conducted.
Pentazocine, when administered orally or parenterally, had no adverse effect on either the reproductive capabilities or the course of pregnancy in rabbits and rats. Embryotoxic effects on the fetuses were not shown.
The daily administration of 4 mg/kg to 20 mg/kg pentazocine subcutaneously to female rats during a 14 day pre-mating period and until the 13th day of pregnancy did not have any adverse effects on the fertility rate.
Pregnancy Teratogenic EffectsPregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Pentazocine hydrochloride and naloxone hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In a published report, a single dose of pentazocine administered to pregnant hamsters on gestation day 8 increased the incidence of exencephaly and cranioschisis at a dose of 196 mg/kg, SC (0.4-times the maximum daily human dose of pentazocine via 12 tablets on a mg/m2 basis).
Animal reproduction studies testing the combination of pentazocine and naloxone during organogenesis have been completed in rats and rabbits. In rats, a pentazocine:naloxone dose of 64 mg/kg:0.64 mg/kg via oral gavage increased the incidences of resorptions and extra ribs (0.2-times the maximum daily human dose of pentazocine via 12 tablets on a mg/m2 basis). There were no clear treatment related effects in rabbits treated with a pentazocine:naloxone dose of up to 64 mg/kg:0.64 mg/kg via oral gavage (0.3-times the maximum daily human dose of pentazocine via 12 tablets on a mg/m2 basis).
Nonteratogenic EffectsThere has been no experience in this regard with pentazocine hydrochloride and naloxone hydrochloride. However, there have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy.
Labor and DeliveryPatients receiving pentazocine during labor have experienced no adverse effects other than those that occur with commonly used analgesics. However, pentazocine can cross the placental barrier and cause central nervous system depression in the newborn and, if used regularly throughout pregnancy, may lead to symptoms of withdrawal in the newborn. pentazocine hydrochloride and naloxone hydrochloride should be used with caution in women delivering premature infants. The effect of pentazocine hydrochloride and naloxone hydrochloride on the mother and fetus, the duration of labor or delivery, the possibility that forceps delivery or other intervention or resuscitation of the newborn may be necessary, or the effect of pentazocine hydrochloride and naloxone hydrochloride on the later growth, development, and functional maturation of the child are unknown at the present time.
Nursing MothersPentazocine is excreted in human milk. Caution should be exercised when pentazocine hydrochloride and naloxone hydrochloride is administered to a nursing woman.
Pediatric UseSafety and effectiveness in pediatric patients below the age of 12 years have not been established.
Geriatric UseControlled clinical studies of pentazocine hydrochloride and naloxone hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses or effectiveness in analgesic activity between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS SECTION
Cardiovascular. Hypertension, hypotension, circulatory depression, tachycardia, syncope.
Respiratory. Rarely, respiratory depression.
Acute CNS Manifestations. Hallucinations (usually visual), disorientation, and confusion .
Other CNS Effects. Grand mal convulsions, increase in intracranial pressure, dizziness, lightheadedness, hallucinations, sedation, euphoria, headache, confusion, disorientation; infrequently weakness, disturbed dreams, insomnia, syncope, and depression; and rarely tremor, irritability, excitement, tinnitus.
Autonomic. Sweating; infrequently flushing; and rarely chills.
Gastrointestinal. Nausea, vomiting, constipation, diarrhea, anorexia, dry mouth, biliary tract spasm, and rarely abdominal distress.
Allergic. Edema of the face; anaphylactic shock; dermatitis, including pruritus; flushed skin, including plethora; infrequently rash, and rarely urticaria.
Ophthalmic. Visual blurring and focusing difficulty, miosis.
Hematologic. Depression of white blood cells (especially granulocytes), with rare cases of agranulocytosis, which is usually reversible, moderate transient eosinophilia.
Dependence and Withdrawal Symptoms. (See WARNINGS, PRECAUTIONS, and DRUG ABUSE AND DEPENDENCE Sections).
Other. Urinary retention, paresthesia, serious skin reactions, including erythema multiforme, Stevens-Johnson syndrome toxic epidermal necrolysis, and alterations in rate or strength of uterine contractions during labor.
OVERDOSAGE SECTION
Manifestations
For pentazocine alone in single doses above 60 mg there have been reports of the occurrence of nalorphine-like psychotomimetic effects such as anxiety, nightmares, strange thoughts, and hallucinations. Somnolence, marked respiratory depression associated with hypertension and tachycardia have also resulted as have seizures, hypotension, dizziness, nausea, vomiting, lethargy, and paresthesias. The respiratory depression is antagonized by naloxone (see Treatment). Circulatory failure and deepening coma may occur in more severe cases, particularly in patients who have also ingested other CNS depressants such as alcohol, sedative/hypnotics, or antihistamines.
Treatment
Adequate measures to maintain ventilation and general circulatory support should be employed. Assisted or controlled ventilation, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Consideration should be given to gastric lavage and gastric aspiration. For respiratory depression due to overdosage or unusual sensitivity to pentazocine, parenteral naloxone is a specific and effective antagonist. Initial doses of 0.4 to 2.0 mg of naloxone are recommended, repeated at 2-3 minute intervals, if needed, up to a total of 10 mg. Anti-convulsant therapy may be necessary.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Adults
The usual initial adult dose is 1 tablet every three or four hours. This may be increased to 2 tablets when needed. Total daily dosage should not exceed 12 tablets.
Discontinuation
Due to the potential for withdrawal symptoms associated with abrupt discontinuation, consideration should be given to tapering patients off pentazocine hydrochloride and naloxone hydrochloride after prolonged periods of treatment with pentazocine hydrochloride and naloxone hydrochloride (See PRECAUTIONS, Drug Abuse and Dependence).
HOW SUPPLIED SECTION
Pentazocine Hydrochloride and Naloxone Hydrochloride Tablets, USP is available as light yellow, capsule shaped tablet debossed “NL” on left side and “680” on the right side of the bisect and plain on the other side. Each tablet contains pentazocine hydrochloride equivalent to 50 mg base and naloxone hydrochloride equivalent to 0.5 mg base.
Bottles of 100 (NDC 43386-680-01).
Store at 20° C -25° C (68° - 77° F); See USP Controlled Room Temperature.
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Distributed by:
GAVIS Pharmaceuticals, LLC
Somerset, NJ 08873
GIN-680-01
Rev: 04/2011