WARNING
Ketoconazole tablets USP, 200 mg should be used only when other effective antifungal therapy is not available or tolerated and the potential benefits are considered to outweigh the potential risks.
Hepatotoxicity
Serious hepatotoxicity, including cases with a fatal outcome or requiring liver transplantation has occurred with the use of oral ketoconazole. Some patients had no obvious risk factors for liver disease. Patients receiving this drug should be informed by the physician of the risk and should be closely monitored. See WARNINGS section.
QT Prolongation and Drug Interactions Leading to QT Prolongation
Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, cisapride. Ketoconazole can cause elevated plasma concentrations of these drugs and may prolong QT intervals, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes. See CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: Drug Interactions sections.
DESCRIPTION
Ketoconazole tablets USP is a synthetic broad-spectrum antifungal agent available in scored white tablets, each containing 200 mg ketoconazole base for oral administration. Inactive ingredients are colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone. Ketoconazole is cis-1-acetyl-4-[4-[[2-(2,4dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxyl]phenyl] piperazine and has the following structural formula:
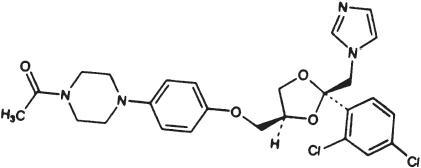
Ketoconazole is a white to slightly beige, odorless powder, soluble in acids, with a molecular weight of 531.44.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Mean peak plasma levels of approximately 3.5 µg/mL are reached within 1 to 2 hours, following oral administration of a single 200 mg dose taken with a meal. Subsequent plasma elimination is biphasic with a half-life of 2 hours during the first 10 hours and 8 hours thereafter. Following absorption from the gastrointestinal tract, ketoconazole is converted into several inactive metabolites. The major identified metabolic pathways are oxidation and degradation of the imidazole and piperazine rings, oxidative Odealkylation and aromatic hydroxylation. About 13% of the dose is excreted in the urine, of which 2 to 4% is unchanged drug. The major route of excretion is through the bile into the intestinal tract. In vitro, the plasma protein binding is about 99% mainly to the albumin fraction. Only a negligible proportion of ketoconazole reaches the cerebrospinal fluid. Ketoconazole is a weak dibasic agent and thus requires acidity for dissolution and absorption.
Electrocardiogram
Pre-clinical electrophysiological studies have shown that ketoconazole inhibits the rapidly activating component of the cardiac delayed rectifier potassium current, prolongs the action potential duration, and may prolong the QTc interval. Data from some clinical PK/PD studies and drug interaction studies suggest that oral dosing with ketoconazole at 200 mg twice daily for 3 to 7 days can result in an increase of the QTc interval: a mean maximum increase of about 6 to 12 msec was seen at ketoconazole peak plasma concentrations about 1 to 4 hours after ketoconazole administration.
MICROBIOLOGY
Mechanism of Action
Ketoconazole blocks the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14α-demethylase responsible for the conversion of lanosterol to ergosterol in the fungal cell membrane. This results in an accumulation of methylated sterol precursors and a depletion of ergosterol within the cell membrane thus weakening the structure and function of the fungal cell membrane.
INDICATIONS AND USAGE
Ketoconazole tablets USP, 200 mg should be used only when other effective antifungal therapy is not available or tolerated and the potential benefits are considered to outweigh the potential risks.
Ketoconazole tablets USP, 200 mg are indicated for the treatment of the following systemic fungal infections in patients who have failed or who are intolerant to other therapies: blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis. Ketoconazole tablets USP, 200 mg should not be used for fungal meningitis because it penetrates poorly into the cerebrospinal fluid.
CONTRAINDICATIONS
Drug Interactions
Coadministration of a number of CYP3A4 substrates is contraindicated with ketoconazole tablets. Coadministration with ketoconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both therapeutic and adverse effects. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia. See WARNINGS section, and PRECAUTIONS: Drug Interactions section for specific examples.
WARNINGS
Ketoconazole tablets USP, 200 mg should be used only when other effective antifungal therapy is not available or tolerated and the potential benefits are considered to outweigh the potential risks.
Hepatotoxicity
Serious hepatotoxicity, including cases with a fatal outcome or requiring liver transplantation, has occurred with the use of oral ketoconazole. Some patients had no obvious risk factors for liver disease. Serious hepatotoxicity was reported both by patients receiving high doses for short treatment durations and by patients receiving low doses for long durations.
The hepatic injury has usually, but not always, been reversible upon discontinuation of ketoconazole tablets treatment. Cases of hepatitis have been reported in children.
At baseline, obtain laboratory tests (such as SGGT, alkaline phosphatase, ALT, AST, total bilirubin (TBL), Prothrombin Time (PT), International Normalization Ratio (INR), and testing for viral hepatitides). Patients should be advised against alcohol consumption while on treatment. If possible, use of other potentially hepatotoxic drugs should be avoided in patients receiving ketoconazole tablets.
Prompt recognition of liver injury is essential. During the course of treatment, serum ALT should be monitored weekly for the duration of treatment. If ALT values increase to a level above the upper limit of normal or 30 percent above baseline, or if the patient develops symptoms, ketoconazole treatment should be interrupted and a full set of liver tests should be obtained. Liver tests should be repeated to ensure normalization of values. Hepatotoxicity has been reported with restarting oral ketoconazole (rechallenge). If it is decided to restart oral ketoconazole, monitor the patient frequently to detect any recurring liver injury from the drug.
QT Prolongation and Drug Interactions Leading to QT Prolongation
Ketoconazole can prolong the QT interval. Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, and cisapride. Ketoconazole can cause elevated plasma concentrations of these drugs which may prolong the QT interval, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes.
Adrenal Insufficiency
Ketoconazole tablets decrease adrenal corticosteroid secretion at doses of 400 mg and higher. This effect is not shared with other azoles. The recommended dose of 200 mg to 400 mg daily should not be exceeded.
Adrenal function should be monitored in patients with adrenal insufficiency or with borderline adrenal function and in patients under prolonged periods of stress (major surgery, intensive care, etc.).
Adverse Reactions Associated with Unapproved Uses
Ketoconazole has been used in high doses for the treatment of advanced prostate cancer and for Cushing's syndrome when other treatment options have failed. The safety and effectiveness of ketoconazole have not been established in these settings and the use of ketoconazole for these indications is not approved by FDA.
In a clinical trial involving 350 patients with metastatic prostatic cancer, eleven deaths were reported within two weeks of starting treatment with high doses of ketoconazole tablets (1200 mg/day). It is not possible to ascertain from the information available whether death was related to ketoconazole therapy or adrenal insufficiency in these patients with serious underlying disease.
Hypersensitivity
Anaphylaxis has been reported after the first dose. Several cases of hypersensitivity reactions including urticaria have also been reported.
Enhanced Sedation
Co-administration of ketoconazole tablets with oral midazolam, oral triazolam or alprazolam has resulted in elevated plasma concentrations of these drugs. This may potentiate and prolong hypnotic and sedative effects, especially with repeated dosing or chronic administration of these agents. Concomitant administration of ketoconazole tablets with oral triazolam, oral midazolam, or alprazolam is contraindicated. (See CONTRAINDICATIONS and PRECAUTIONS: Drug Interactions sections.)
Myopathy
Co-administration of CYP3A4 metabolized HMG-CoA reductase inhibitors such as simvastatin, and lovastatin is contraindicated with ketoconazole tablets. (See CONTRAINDICATIONS and PRECAUTIONS: Drug Interactions sections.)
PRECAUTIONS
General
Ketoconazole tablets have been demonstrated to lower serum testosterone. Once therapy with ketoconazole tablets has been discontinued, serum testosterone levels return to baseline values. Testosterone levels are impaired with doses of 800 mg per day and abolished by 1600 mg per day. Clinical manifestations of decreased testosterone concentrations may include gynecomastia, impotence and oligospermia.
Information for Patients
Patients should be instructed to report any signs and symptoms which may suggest liver dysfunction so that appropriate biochemical testing can be done. Such signs and symptoms may include unusual fatigue, anorexia, nausea and/or vomiting, abdominal pain, jaundice, dark urine or pale stools (see WARNINGS section).
Drug Interactions
Drugs that affect the absorption, distribution, metabolism, and excretion of ketoconazole may alter the plasma concentrations of ketoconazole. For example, gastric acid suppressants (e.g., antacids, histamine H2-blockers, proton pump inhibitors) have been shown to reduce plasma concentrations of ketoconazole.Ketoconazole is a substrate and potent inhibitor of CYP3A4. Therefore, the following drug interactions may occur when ketoconazole is co-administered with other drugs that interact with CYP3A4. (See Table 1 and Table 2 for an overview of these drug interactions; details are provided in the text that follows these tables.)
- Ketoconazole may decrease the elimination of drugs metabolized by CYP3A4, thereby increasing their plasma concentrations. Increased exposure to these drugs may cause an increase or prolongation of their therapeutic and/or adverse effects. Concomitant use with ketoconazole tablets is contraindicated for drugs known to present a risk of serious side effects with increased exposure (see BOXED WARNING, CONTRAINDICATIONS section, and PRECAUTIONS: Drug Interactions, Table 1). For others, monitoring of plasma concentrations is advised when possible. Clinical signs and symptoms associated with these drugs should be monitored, with dosage adjusted as needed.
- Inducers of CYP3A4 may decrease the plasma concentrations of ketoconazole (see Table 2). Ketoconazole may not be effective in patients concomitantly taking one of these drugs. Therefore, administration of these drugs with ketoconazole is not recommended.
- Other inhibitors of CYP3A4 may increase the plasma concentrations of ketoconazole (see Table 2). Patients who must take ketoconazole concomitantly with one of these drugs should be monitored closely for signs or symptoms of increased or prolonged pharmacologic effects of ketoconazole.
|
|
| Systemic exposure to these drugs is increased significantly by the addition of ketoconazole: Concomitant use with ketoconazole is contraindicated. | |
| Alprazolam, midazolam, triazolam | HMG-CoA reductase inhibitors (lovastatin, simvastatin) |
| Cisapride | Nisoldipine |
| Dofetilide | Pimozide |
| Eplerenone | Quinidine |
| Ergot alkaloids (ergotamine, dihydroergotamine) | |
| Systemic exposure to these drugs is increased by ketoconazole: Careful monitoring, with possible adjustment in dosage, is recommended. | |
| Alfentanil, fentanyl, sulfentanil | Indinavir, saquinavir |
| Amlodipine, felodipine, nicardipine, nifedipine | Methylprednisolone |
| Bosentan | Rifabutin |
| Buspirone | Sildenafil |
| Busulfan | Sirolimus (co-administration not recommended) |
| Carbamazepine | Tacrolimus |
| Cilostazol | Telithromycin |
| Cyclosporine | Tolterodine |
| Digoxin | Trimetrexate |
| Docetaxel, paclitaxel | Verapamil |
| Oral anti-coagulants | Vinca alkaloids (vincristine, - vinblastine, vinorelbine) |
| *This list is not all-inclusive. | |
| Systemic exposure to ketoconazole is reduced significantly by these drugs: Concomitant use with ketoconazole is not recommended. | |
| Carbamazepine | Phenytoin |
| Gastric Acid Suppressants (antacids, antimuscarinics, histamine H2-blockers, proton pump inhibitors, sucralfate) | Rifampin, rifabutin, isoniazid |
| Nevirapine | |
| Systemic exposure to ketoconazole is increased significantly by this drug: Dose reduction of ketoconazole should be considered | |
| Ritonavir | |
1. Effects of ketoconazole on other drugs
1.1 Systemic exposure to the following drugs is significantly increased by coadministration of ketoconazole. Concomitant use of these drugs with ketoconazole tablets USP, 200 mg is contraindicated
Alprazolam, midazolam, triazolam
Co-administration of ketoconazole tablets with alprazolam, midazolam, or triazolam has resulted in elevated plasma concentrations of these drugs. This may potentiate and prolong hypnotic and sedative effects, especially with repeated or chronic administration of these agents. Concomitant administration of ketoconazole tablets with alprazolam, oral midazolam, and oral triazolam is contraindicated. (See CONTRAINDICATIONS and WARNINGS sections.) Special precaution and patient monitoring are required with concomitant parenteral midazolam, because the sedative effect may be prolonged.
Cisapride
Oral ketoconazole potently inhibits the metabolism of cisapride resulting in a mean eight-fold increase in AUC of cisapride, which can lead to prolongation of QT interval. Therefore concomitant administration of ketaconazole tablets with cisapride is contraindicated. (See BOXED WARNING, CONTRAINDICATIONS, and WARNINGS sections.)
Dofetilide
The class III antiarrhythmic dofetilide is known to prolong the QT interval. The potential increase in dofetilide plasma concentrations when administered concomitantly with ketoconazole could result in serious cardiovascular events including QTc prolongation and rare occurrences of torsades de pointes. Therefore, concomitant administration of ketoconazole tablets with dofetilide is contraindicated. (See BOXED WARNING, CONTRAINDICATIONS, and WARNINGS sections.)
Eplerenone
Ketoconazole increases the eplerenone AUC by roughly 5-fold, thereby increasing the risk for hyperkalemia and hypotension. Co-administration of ketoconazole and eplerenone is contraindicated. (See CONTRAINDICATIONS section.)
Ergot Alkaloids
Elevated concentrations of ergot alkaloids can cause ergotism, i.e., a risk for vasospasm potentially leading to cerebral ischemia and/or ischemia of the extremities. Concomitant administration of ergot alkaloids such as dihydroergotamine and ergotamine with ketoconazole tablets is contraindicated. (See CONTRAINDICATIONS section.)
HMG-CoA Enzyme Inhibitors (lovastatin, simvastatin)
Co-administration of ketoconazole with CYP3A4-metabolized HMG-CoA reductase inhibitors such as simvastatin, and lovastatin, may increase the risk of skeletal muscle toxicity, including rhabdomyolysis. Concomitant administration of ketoconazole tablets with these HMG-CoA reductase inhibitors is contraindicated. (See CONTRAINDICATIONS and WARNINGS sections.)
Nisoldipine
Pre-treatment with and concomitant administration of ketoconazole resulted in a 24-fold and 11-fold increase in mean AUC and Cmax of nisoldipine, respectively, compared with treatment with nisoldipine 5 mg alone. Concomitant administration of ketoconazole with nisoldipine is contraindicated. (See CONTRAINDICATIONS section.)
Pimozide
Pimozide is known to prolong the QT interval and is partially metabolized by CYP3A4. Co-administration of ketoconazole and pimozide could result in serious cardiovascular events including QTc prolongation and rare occurrences of torsades de pointes, and is therefore contraindicated. (See BOXED WARNING, CONTRAINDICATIONS, and WARNINGS sections.)
Quinidine
The class IA antiarhythmic quinidine is known to prolong the QT interval. The potential increase in quinidine plasma concentrations when administered concomitantly with ketoconazole could result in serious cardiovascular events including QTc prolongation and rare occurrences of torsades de pointes. Therefore, concomitant administration of ketoconazole tablets with quinidine is contraindicated. (See BOXED WARNING, CONTRAINDICATIONS, and WARNINGS sections.)
1.2 Co-administration of ketoconazole with the following agents was shown or is expected to result in increased exposure to these drugs. Therefore, careful monitoring of plasma concentrations or adverse events of these drugs is recommended. Adjustment of dosage of these drugs may be needed
Alfentanil, sufentanil, fentanyl
In vitro data suggest that alfentanil, sufentanil and fentanyl are metabolized by CYP3A4. Concomitant administration of ketoconazole tablets and alfentanil, sufentanil, or fentanyl may increase plasma concentrations of the latter drugs.
Amlodipine, felodipine, nicardipine, nifedipine
CYP3A4 metabolized calcium channel blockers such as amlodipine, felodipine, nicardipine, and nifedipine should be used cautiously with ketoconazole tablets as ketoconazole may cause several-fold increases in plasma concentrations of these calcium channel blockers.
Bosentan
Concomitant administration of ketoconazole increased the Cmax and AUC of bosentan 2.1- and 2.3 – fold, respectively. No dosage adjustment of bosentan is needed but close monitoring for increased bosentan-associated adverse effects is recommended.
Buspirone
Concomitant administration of buspirone with ketoconazole may result in significant increases in plasma concentrations of buspirone. When administered with ketoconazole tablets, a low initial dose of buspirone with subsequent dosage adjustment based on clinical assessment is recommended.
Busulfan
Ketoconazole tablets may decrease the clearance and thus increase the systemic exposure to busulfan.
Carbamazepine
In vivo studies have demonstrated an increase in plasma carbamazepine concentrations in subjects concomitantly receiving ketoconazole. Close monitoring of plasma carbamazepine concentrations is recommended whenever ketoconazole is given to patients stabilized on carbamazepine therapy.
Cilostazol
Ketoconazole had been shown to increase both cilostazol AUC and Cmax by about two-fold when administered concurrently. Co-administration of ketoconazole with cilostazol resulted in increased incidences of adverse effects, such as headache. When ketoconazole tablets is administered concomitantly with cilostazol, the prescriber should consider up to a 50% reduction in cilostazol dosage.
Cyclosporine
Ketoconazole tablets may alter the metabolism of cyclosporine, thereby resulting in elevated cyclosporine plasma concentrations. Dosage adjustment may be required if cyclosporine or tacrolimus is given concomitantly with ketoconazole tablets.
Digoxin
Rare cases of elevated plasma concentrations of digoxin have been reported. It is not clear whether this was due to the combination of therapy. It is, therefore, advisable to monitor digoxin concentrations in patients receiving ketoconazole.
Docetaxel
In the presence of ketoconazole, the clearance of docetaxel in cancer patients was shown to decrease by 50%. When docetaxel and ketoconazole are administered together, dosage reduction in docetaxel may be necessary in order to minimize the incidence of toxicities associated with docetaxel.
Indinavir, saquinavir
Concomitant administration of ketoconazole and protease inhibitors metabolized by CYP3A4, such as indinavir and saquinavir, may increase plasma concentrations of these protease inhibitors. Dosage reduction of indinavir is recommended when administering ketoconazole concomitantly. No dosage adjustments are recommended when saquinavir and ketoconazole are coadministered for a short period of time.
Methylprednisolone
Ketoconazole tablets may alter the metabolism of methylprednisolone, resulting in elevated plasma concentrations of methylprednisolone. Dose adjustments may be required if methylprednisolone is given concomitantly with ketoconazole tablets.
Oral anti-coagulants
Oral imidazole compounds such as ketoconazole may enhance the anticoagulant effect of coumarin-like drugs, thus the anticoagulant effect should be carefully titrated and monitored.
Oral hypoglycemic agents
Because severe hypoglycemia has been reported in patients concomitantly receiving oral miconazole (an imidazole) and oral hypoglycemic agents, such a potential interaction involving the latter agents when used concomitantly with ketoconazole tablets (an imidazole) cannot be ruled out.
Rifabutin
Ketoconazole was shown to inhibit the CYP-mediated metabolism of rifabutin in vitro. Co-administration with ketoconazole tablets may result in elevated plasma concentrations of rifabutin.
Sildenafil
Ketoconazole had been shown to increase sildenafil plasma concentrations. When used concomitantly with ketoconazole tablets, a 50% reduction in sildenafil starting dose should be considered.
Sirolimus
Multiple-dose ketoconazole had been shown to increase sirolimus Cmax and AUC by 4.3-fold and 10.9-fold, respectively. The concomitant use of ketoconazole tablets and sirolimus is not recommended.
Tacrolimus
Ketoconazole had been shown to decrease the oral clearance of tacrolimus thereby leading to a 2-fold increase in tacrolimus oral bioavailability. Adjustment in tacrolimus dosage may be required if tacrolimus is given concomitantly with ketoconazole tablets.
Telithromycin
Ketoconazole increased the AUC of telithromycin by 1.5 to 2-fold. Use caution when administering telithromycin concurrently with ketoconazole tablets since this may result in an increased risk for telithromycin associated adverse events.
Tolterodine
In the presence of ketoconazole, the apparent oral clearance of tolterodine decreased resulting in at least a two-fold increase in tolterodine. For patients receiving ketoconazole, a 50% reduction in the initial tolterodine dosage is recommended.
Trimetrexate
In vitro data suggest that trimetrexate is extensively metabolized by CYP3A4. In vitro animal models have demonstrated that ketoconazole potently inhibits the metabolism of trimetrexate. Patients treated concomitantly with trimetrexate and ketoconazole tablets should be carefully monitored for trimetrexate-associated toxicities.
2. Effects of other drugs on ketoconazole
2.1 Drugs affecting the absorption of ketoconazole
Gastric Acid Suppressors/Neutralizers
Studies have shown that absorption of ketoconazole is impaired when gastric acid production is decreased. Reduced plasma concentrations of ketoconazole were reported when ketoconazole tablets were administered with antacids, antimuscarinics, histamine H2-blockers, proton pump inhibitors (omeprazole, lansoprazole) and sucralfate. (See PRECAUTIONS, Drug Interactions (General) section.)
2.2 Drugs that were shown or are expected to significantly reduce the systemic exposure to ketoconazole
Co-administration of ketoconazole with potent CYP3A4 enzyme inducers is not recommended.
Carbamazepine
Concomitant administration of ketoconazole tablets with carbamazepine may alter the metabolism of one or both of the drugs. Close monitoring for both plasma concentrations of carbamazepine and reduced ketoconazole efficacy is recommended.
Nevirapine
Ketoconazole AUC and Cmax decreased by a median of 63% and 40%, respectively, in HIV-infected patients who were given nevirapine 200 mg once daily for two weeks along with ketoconazole 400 mg daily. Concomitant administration of ketoconazole tablets and nevirapine is not recommended.
Phenytoin
Concomitant administration of ketoconazole with phenytoin may alter the metabolism of one or both of the drugs. Close monitoring for both plasma concentrations of phenytoin and reduced efficacy of ketoconazole tablets is recommended.
Rifampin, rifabutin, isoniazid
Concomitant administration of rifampin and rifabutin with ketoconazole tablets reduces the blood concentrations of the latter. INH (Isoniazid) was also reported to affect ketoconazole concentrations adversely. These antitubercular drugs should not be given concomitantly with ketoconazole tablets.
3. Other drug interactions
Alcohol
Rare cases of a disulfiram-like reaction to alcohol have been reported. These experiences have been characterized by flushing, rash, peripheral edema, nausea, and headache. Symptoms resolved within a few hours.
Loratadine
After the co-administration of 200 mg oral ketoconazole twice daily and one 20 mg dose of loratadine to 11 subjects, the AUC and Cmax of loratadine averaged 302% (±142 S.D.) and 251% (± 68 S.D.), respectively, of those obtained after cotreatment with placebo. The AUC and Cmax of descarboethoxyloratadine, an active metabolite, averaged 155% (± 27 S.D.) and 141% (± 35 S.D.), respectively. However, no related changes were noted in the QTc on ECG taken at 2, 6, and 24 hours after the coadministration. Also, there were no clinically significant differences in adverse events when loratadine was administered with or without ketoconazole.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Ketoconazole did not show any signs of mutagenic potential when evaluated using the dominant lethal mutation test or the Ames Salmonella microsomal activator assay. Ketoconazole was not carcinogenic in an 18-month, oral study in Swiss albino mice or a 24-month oral carcinogenicity study in Wistar rats at dose levels of 5, 20 and 80 mg/kg/ day. The high dose in these studies was approximately 1× (mouse) or 2× (rat) the clinical dose in humans based on a mg/m2 comparison.
Pregnancy
Teratogenic effects
Pregnancy Category C
Ketoconazole has been shown to be teratogenic (syndactylia and oligodactylia) in the rat when given in the diet at 80 mg/kg/day (2 times the maximum recommended human dose, based on body surface area comparisons). However, these effects may be related to maternal toxicity, evidence of which also was seen at this and higher dose levels.
There are no adequate and well controlled studies in pregnant women. Ketoconazole tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Ketoconazole has also been found to be embryotoxic in the rat when given in the diet at doses higher than 80 mg/kg during the first trimester of gestation.
In addition, dystocia (difficult labor) was noted in rats administered oral ketoconazole during the third trimester of gestation. This occurred when ketoconazole was administered at doses higher than 10 mg/kg (about one fourth the maximum human dose, based on body surface area comparison).
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions were reported in clinical trials:
Immune System Disorders: anaphylactoid reaction
Endocrine Disorders: gynecomastia
Metabolism and Nutrition Disorders: alcohol intolerance, anorexia, hyperlipidemia, increased appetite
Psychiatric Disorders: insomnia, nervousness
Nervous System Disorders: headache, dizziness, paresthesia, somnolence
Eye Disorders: photophobia
Vascular Disorders: orthostatic hypotension
Respiratory, Thoracic and Mediastinal Disorders: epistaxis
Gastrointestinal Disorders: vomiting, diarrhea, nausea, constipation, abdominal pain, abdominal pain upper, dry mouth, dysgeusia, dyspepsia, flatulence, tongue discoloration
Hepatobiliary Disorders: hepatitis, jaundice, hepatic function abnormal
Skin and Subcutaneous Tissues Disorders: erythema multiforme, rash, dermatitis, erythema, urticaria, pruritus, alopecia, xeroderma
Musculoskeletal and Connective Tissue Disorders: myalgia
Reproductive System and Breast Disorders: menstrual disorder
General Disorders and Administration Site Conditions: asthenia, fatigue, hot flush, malaise, edema peripheral, pyrexia, chills
Investigations: platelet count decreased.
Post-Marketing Experience
The following adverse reactions have been identified during postapproval use of ketoconazole tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions were reported during post-marketing experience:
Blood and Lymphatic System Disorders: thrombocytopenia
Immune System Disorders: allergic conditions including anaphylactic shock, anaphylactic reaction, angioneurotic edema
Endocrine Disorders: adrenocortical insufficiency
Nervous System Disorders: reversible intracranial pressure increased (e.g. papilloedema, fontanelle bulging in infants)
Hepatobiliary Disorders: serious hepatotoxicity including hepatitis cholestatic, biopsyconfirmed hepatic necrosis, cirrhosis, hepatic failure including cases resulting in transplantation or death
Skin and Subcutaneous Tissue Disorders: acute generalized exanthematous pustulosis, photosensitivity
Musculoskeletal and Connective Tissue Disorders: arthralgia
Reproductive System and Breast Disorders: erectile dysfunction; with doses higher than the recommended therapeutic dose of 200 or 400mg daily, azoospermia.
OVERDOSAGE
In the event of acute accidental overdose, treatment consists of supportive and symptomatic measures. Within the first hour after ingestion, activated charcoal may be administered.
DOSAGE AND ADMINISTRATION
There should be laboratory as well as clinical documentation of infection prior to starting ketoconazole therapy. The usual duration of therapy for systemic infection is 6 months. Treatment should be continued until active fungal infection has subsided.
HOW SUPPLIED
Ketoconazole tablets USP, 200 mg are white to off-white round, flat tablets, one side scored and engraved with "T" above the score and "57" below the score. The other side is plain. They are supplied in bottles of 30 tablets (NDC 33261-0739-30).
Mfd. by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel 26110
Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532
Repackaged By :
Aidarex Pharmaceuticals LLC,
Corona, CA 92880
Revised: August, 2013
79775-0813-3
MEDICATION GUIDE
Ketoconazole Tablets USP, 200 mg
What is the most important information I should know about ketoconazole tablets USP?
Ketoconazole tablets is not the only medicine available to treat fungal infections and should only be used when other medicines are not right for you. Talk to your healthcare provider to find out if ketoconazole tablets are right for you.
Ketoconazole tablets USP can cause serious side effects, including:
-
liver problems (hepatotoxicity). Some people who were treated with ketoconazole the active ingredient in ketoconazole tablets, had serious liver problems that led to death or the need for a liver transplant. Call your healthcare provider right away if you have any of the following symptoms:
- loss of appetite or start losing weight (anorexia)
- nausea or vomiting
- feel tired
- stomach pain or tenderness
- dark urine or light colored stools
- yellowing of your skin or the whites of your eyes
- fever or rash
- changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heart beats that can be life threatening. This can happen when ketoconazole tablets are taken with certain medicines, such as dofetilide, quinidine, pimozide, and cisapride. Talk to your healthcare provider about other medicines you are taking before you start taking ketoconazole tablets. Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast. These may be symptoms related to QT prolongation.
What are ketoconazole tablets USP?
- Ketoconazole tablets are prescription medicine used to treat serious fungal infections including: blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis.
- Ketoconazole tablets are not for people with fungal nail infections.
- Ketoconazole tablets have not been approved for the treatment of advanced prostate cancer or Cushing's syndrome. The safety and efficacy have not been established.
- Ketoconazole tablets should only be used in children if prescribed by the healthcare provider who has determined that the benefits outweigh the risks.
Who should not take ketoconazole tablets USP?
-
Do not take ketoconazole tablets if you:
- have liver problems
- take simvastatin, and lovastatin. Ketoconazole tablets when taken with these medicines may cause muscle problems.
- take eplerenone, dihydroergotamine, ergotamine, and nisoldipine.
- take triazolam, midazolam, or alprazolam. Taking ketoconazole tablets with these medicines may make you very drowsy and make your drowsiness last longer.
- are allergic to ketoconazole or any of the ingredients in ketoconazole tablets. See the end of this Medication Guide for a complete list of ingredients in ketoconazole tablets.
Before you take ketoconazole tablets USP, tell your healthcare provider if you:
- have had an abnormal heart rhythm tracing (ECG) or anyone in your family have or have had a heart problem called "congenital long QT syndrome".
- have adrenal insufficiency.
- are pregnant or plan to become pregnant. It is not known if ketoconazole tablets will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Ketoconazole tablets can pass into your breast milk. You and your healthcare provider should decide if you will take ketoconazole tablets or breastfeed. You should NOT do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using ketoconazole tablets with certain other medicines may affect each other. Using ketoconazole tablets with other medicines can cause serious side effects.
How should I take ketoconazole tablets USP?
- Take ketoconazole tablets 1 time each day.
- Do not stop taking ketoconazole tablets without first talking to your healthcare provider.
What should I avoid while taking ketoconazole tablets USP?
- Do not drink alcohol while taking ketoconazole tablets.
What are the possible side effects of ketoconazole tablets USP?
Ketoconazole tablets may cause serious side effects, including:
- See "What is the most important information I should know about ketoconazole tablets USP?"
- adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Ketoconazole tablets may cause adrenal insufficiency if you take a high dose. Your healthcare provider will follow you closely if you have adrenal insufficiency or if you are taking prednisone or other similar medicines for long periods of time. Call your healthcare provider right away if you have symptoms of adrenal insufficiency such as tiredness, weakness, dizziness, nausea, and vomiting.
- serious allergic reactions. Some people can have a serious allergic reaction to ketoconazole tablets. Stop taking ketoconazole tablets and go to the nearest hospital emergency room right away if you get a rash, itching, hives, fever, swelling of the lips or tongue, chest pain, or have trouble breathing. These could be signs of a serious allergic reaction.
- muscle problems. Taking certain medicines with ketoconazole tablets may cause muscle problems. See "Who should not take ketoconazole tablets USP?"
The most common side effects of ketoconazole tablets include nausea, headache, diarrhea, stomach pain, and abnormal liver function tests.
These are not all the possible side effects of ketoconazole tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ketoconazole tablets USP?
- Store ketoconazole tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep ketoconazole tablets dry.
General information about the safe and effective use of ketoconazole tablets USP.
Medications are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ketoconazole tablets for a condition for which it was not prescribed. Do not give ketoconazole tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about ketoconazole tablets.
If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about ketoconazole tablets that is written for health professionals.
What are the ingredients in ketoconazole tablets USP, 200 mg?
Active ingredient: ketoconazole.
Inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone.
This Medication Guide has been approved by the U.S. Food and Drug Administration
