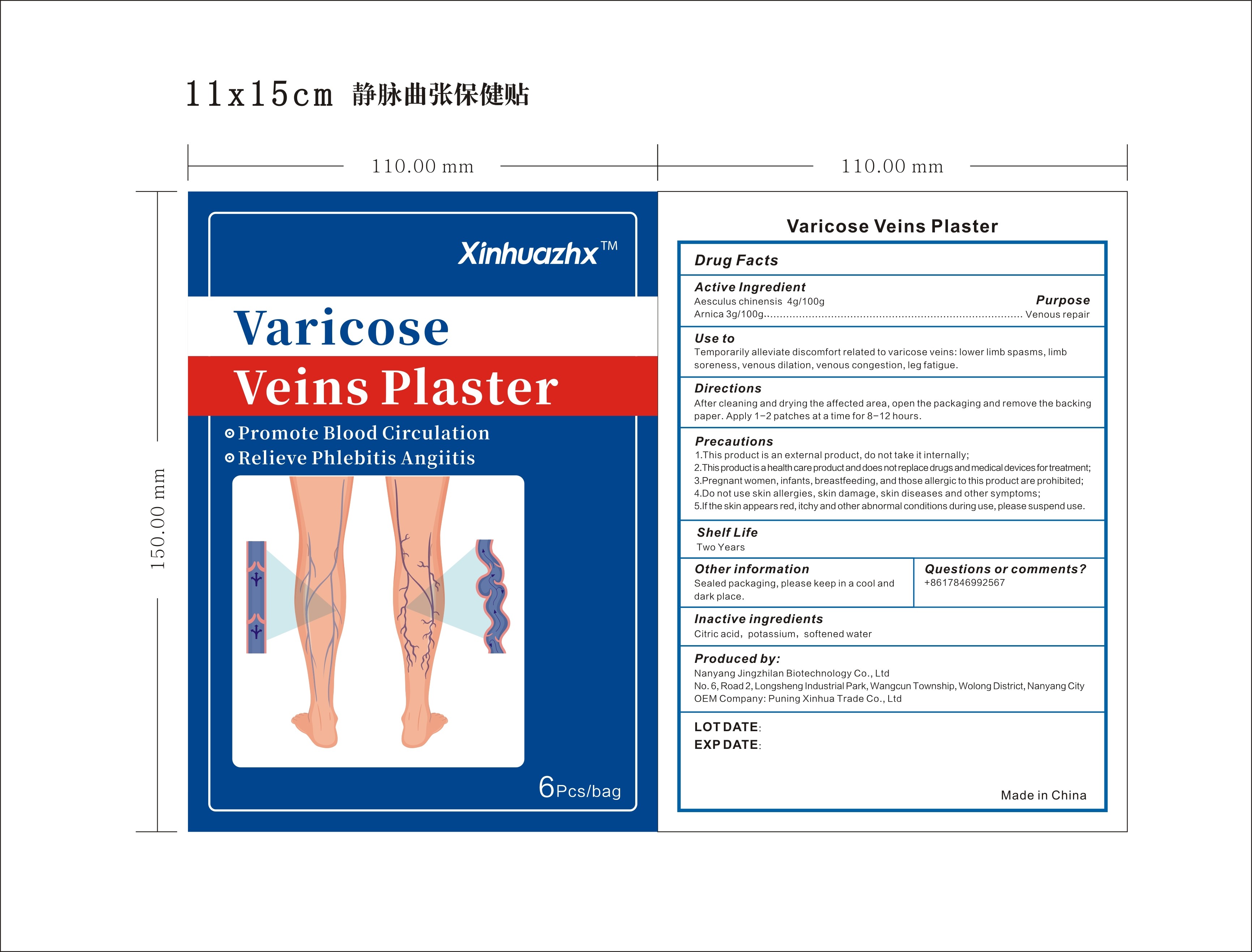
Use to: Temporarily alleviate discomfort related to varicose veins: lower limb spasms, limb soreness, venous dilation, venous congestion, leg fatigue.
Directions: After cleaning and drying the affected area, open the packaging and remove the backing paper. Apply 1-2 patches at a time for 8-12 hours.
Precautions:
1.This product is an external product, do not take it internally;
2.This product is a health care product and does not replace drugs and medical devices fortreatment;
3.Pregnant women, infants, breastfeeding, and those allergic to this product are prohibited;
4.Do not use skin allergies, skin damage, skin diseases and other symptoms;
5.If the skin appears red, itchy and other abnormal conditions during use, please suspend use.
 Label
Label