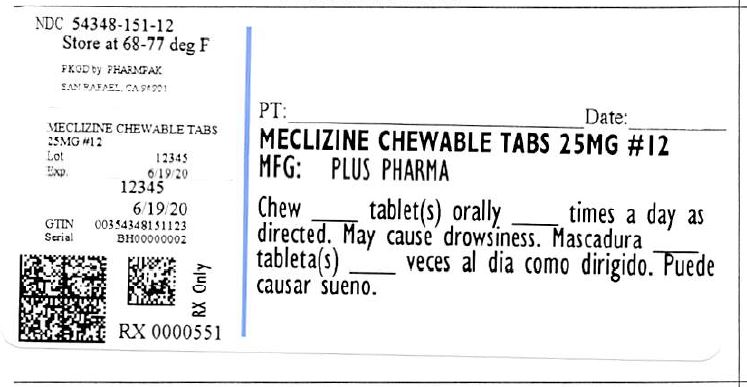
MECLIZINE HCL 25 MG- meclizine hydrochloride tablet, chewable
Pharmpak, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Meclizine HCl 25 mg Chewable Tablets
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Keep out of reach of children.
In case of overdose, get medical help or contact the poison control center immediately.
Directions
- Dosage should be taken one hour before travel starts.
- Adults and children 12 years of age and older: Chew 1-2 tablets once daily or as directed by a doctor
- Children under 12 years: do not give this product to children under 12 years of age unless directed by a doctor.
- Phenylketonurics: Contains phenylalanine 0.28 mg per tablet
- Do not use if imprinted safety seal under cap is broken or missing
Inactive ingredients
aspartame, croscarmellose sodium, dextrose, FD&C Red #40 Lake, magnesium stearate, maltodextrin, microcrystalline cellulose, natural and artificial flavors, silicon dioxide, sodium sulfate, sugar, tricalcium phosphate.
Questions or comments?
If you have any questions or comments or to report an adverse event, please contact (800) 795-9775.
Distributed by: Plus Pharma, Commack, NY 11725
*Plus Pharma is not affiliated with the owner of the registered trademark Bonine®.
| MECLIZINE HCL 25 MG
meclizine hydrochloride tablet, chewable |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Pharmpak, Inc. (175493840) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmpak, Inc. | 175493840 | repack(54348-151) | |