FULL PRESCRIBING INFORMATION
WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS Savella is a selective serotonin and norepinephrine reuptake inhibitor (SNRI), similar to some drugs used for the treatment of depression and other psychiatric disorders. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of such drugs in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on Savella should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Savella is not approved for use in the treatment of major depressive disorder. Savella is not approved for use in pediatric patients [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)]
1 INDICATIONS AND USAGE
Savella is indicated for the management of fibromyalgia.
Savella is not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
2 DOSAGE AND ADMINISTRATION
Savella is given orally with or without food.
Taking Savella with food may improve the tolerability of the drug.
2.1 Recommended Dosing
The recommended dose of Savella is 100 mg/day (50 mg twice daily).
Based on efficacy and tolerability dosing may be titrated according to the following schedule:
Day 1: 12.5 mg once
Days 2-3: 25 mg/day (12.5 mg twice daily)
Days 4-7: 50 mg/day (25 mg twice daily)
After Day 7: 100 mg/day (50 mg twice daily)
Based on individual patient response, the dose may be increased to 200 mg/day (100 mg twice daily).
Doses above 200 mg/day have not been studied.
Savella should be tapered and not abruptly discontinued after extended use [see Discontinuing Savella (2.4) and Warnings and Precautions (5.7)]
2.2 Patients with Renal Insufficiency
No dosage adjustment is necessary in patients with mild renal impairment. Savella should be used with caution in patients with moderate renal impairment. For patients with severe renal impairment (indicated by an estimated creatinine clearance of 5-29 mL/min), the maintenance dose should be reduced by 50% to 50 mg/day (25 mg twice daily).
Based on individual patient response, the dose may be increased to 100 mg/day (50 mg twice daily).
Savella is not recommended for patients with end-stage renal disease.
2.3 Patients with Hepatic Insufficiency
No dosage adjustment is necessary for patients with hepatic impairment.
As with any drug, caution should be exercised in patients with severe hepatic impairment.
2.4 Discontinuing Savella
Withdrawal symptoms have been observed in clinical trials following discontinuation of milnacipran, as with other serotonin and norepinephrine re-uptake inhibitors (SNRIs) and selective serotonin re-uptake inhibitors (SSRIs). Patient should be monitored for these symptoms when discontinuing treatment. Savella should be tapered and not abruptly discontinued after extended use [see Warnings and Precautions (5.7)].
2.5 Switching patients to or from a Monoamine Oxidase Inhibitor (MAOI)
At least 14 days should elapse between discontinuation of a MAOI and initiation of therapy with Savella. In addition, at least 5 days should be allowed after stopping Savella before starting a MAOI [see Contraindications (4.1)].
3 DOSAGE FORMS AND STRENGTHS
Film-coated, immediate release tablets in four strengths: 12.5 mg, 25 mg, 50 mg, and 100 mg of milnacipran hydrochloride.
12.5 mg tablets are round, pink, "F" on one side, "L" on the reverse;
25 mg tablets are round, white, "FL" on one side, "25" on the reverse;
50 mg tablets are oval, green, "FL" on one side, "50" on the reverse;
100 mg tablets are oval, blue, "FL" on one side, "100" on the reverse
[see Description (11) and How Supplied/ Storage and Handling (16)].
4 CONTRAINDICATIONS
4.1 Monoamine Oxidase Inhibitors
Concomitant use of Savella in patients taking monoamine oxidase inhibitors (MAOIs) is contraindicated. In patients receiving a serotonin reuptake inhibitor in combination with a monoamine oxidase inhibitor (MAOI), there have been reports of serious, sometimes fatal, reactions including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have recently discontinued serotonin reuptake inhibitors and have been started on an MAOI. Some cases presented with features resembling neuroleptic malignant syndrome. The effects of combined use of Savella and MAOIs have not been evaluated in humans. Therefore, it is recommended that Savella should not be used in combination with an MAOI, or within 14 days of discontinuing treatment with an MAOI. Similarly, at least 5 days should be allowed after stopping Savella before starting an MAOI [see Dosage and Administration (2.5), Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Suicide Risk
Savella is a selective serotonin and norepinephrine re-uptake inhibitor (SNRI), similar to some drugs used for the treatment of depression and other psychiatric disorders.
Patients, both adult and pediatric, with depression or other psychiatric disorders may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking these medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants, including drugs that inhibit the reuptake of norepinephrine and/or serotonin, may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
In the placebo-controlled clinical trials of adults with fibromyalgia, among the patients who had a history of depression at treatment initiation, the incidence of suicidal ideation was 0.5% in patients treated with placebo, 0% in patients treated with Savella 100 mg/day, and 1.3% in patients treated with Savella 200 mg/day. No suicides occurred in the short-term or longer-term (up to 1 year) fibromyalgia trials.
Pooled analyses of short-term placebo-controlled trials of drugs used to treat depression (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with these drugs compared to placebo in adults beyond age 24; there was a reduction in suicidality risk with antidepressants compared to placebo in adults age 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 drugs used to treat depression in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk of differences (drug versus placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
| < 18 | 14 additional cases |
| 18-24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25-64 | 1 fewer case |
| ≥ 65 | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with drugs inhibiting the reuptake of norepinephrine and/or serotonin for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, have been reported in adult and pediatric patients being treated with drugs inhibiting the reuptake of norepinephrine and/or serotonin for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients who may experience worsening depressive symptoms, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe or abrupt in onset, or were not part of the patient's presenting symptoms.
If the decision has been made to discontinue treatment due to worsening depressive symptoms or emergent suicidality, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can produce withdrawal symptoms [see Dosage and Administration—Recommended Dosing (2.1), Dosage—Discontinuing Savella (2.4), and Warnings and Precautions—Discontinuation of Treatment with Savella (5.7)].
Families and caregivers of patients being treated with drugs inhibiting the reuptake of norepinephrine and/or serotonin for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for Savella should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
5.2 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions
The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and SSRIs alone, including Savella, but particularly with concomitant use of serotonergic drugs (including triptans), with drugs which impair metabolism of serotonin (including MAOIs) or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea) [see Drug Interactions (7)]. Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
The concomitant use of Savella with MAOIs is contraindicated [see Contraindications (4.1)].
If concomitant treatment of Savella with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see Drug Interactions (7)].
The concomitant use of Savella with serotonin precursors (such as tryptophan) is not recommended [see Drug Interactions (7)]. Treatment with Savella and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
5.3 Effects on Blood Pressure
Inhibition of the reuptake of norepinephrine (NE) and serotonin (5-HT) can lead to cardiovascular effects. SNRIs, including Savella, have been associated with reports of increase in blood pressure.
In a double-blind, placebo-controlled clinical pharmacology study in healthy subjects designed to evaluate the effects of milnacipran on various parameters, including blood pressure at supratherapeutic doses, there was evidence of mean increases in supine blood pressure at doses up to 300 mg twice daily (600 mg/day). At the highest 300 mg twice daily dose, the mean increase in systolic blood pressure was up to 8.1 mm Hg for the placebo group and up to 10.0 mm Hg for the Savella treated group over the 12 hour steady state dosing interval. The corresponding mean increase in diastolic blood pressure over this interval was up to 4.6 mm Hg for placebo and up to 11.5 mm Hg for the Savella treated group.
In the 3-month placebo-controlled fibromyalgia clinical trials, Savella treatment was associated with mean increases of up to 3.1 mm Hg in systolic blood pressure (SBP) and diastolic blood pressure (DBP) [see Adverse Reactions (6.3)].
In the placebo-controlled trials, among fibromyalgia patients who were non-hypertensive at baseline, approximately twice as many patients in the Savella treatment arms became hypertensive at the end of the study (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) compared with the placebo patients: 7.2% of patients in the placebo arm versus 19.5% of patients treated with Savella 100 mg/day and 16.6% of patients treated with Savella 200 mg/day. Among patients who met systolic criteria for pre-hypertension at baseline (SBP 120-139 mmHg), more patients became hypertensive at the end of the study in the Savella treatment arms than placebo: 9% of patients in the placebo arm versus 14% in both the Savella 100 mg/day and the Savella 200 mg/day treatment arms.
Among fibromyalgia patients who were hypertensive at baseline, more patients in the Savella treatment arms had a >15 mmHg increase in SBP than placebo at the end of the study: 1% of patients in the placebo arm versus 7% in the Savella 100 mg/day and 2% in the Savella 200 mg/day treatment arms. Similarly, more patients who were hypertensive at baseline and were treated with Savella had DBP increases > 10 mmHg than placebo at the end of study: 3% of patients in the placebo arm versus 8% in the Savella 100 mg/day and 6% in the Savella 200 mg/day treatment arms.
Sustained increases in SBP (increase of ≥ 15 mmHg on three consecutive post-baseline visits) occurred in 2% of placebo patients versus 9% of patients receiving Savella 100 mg/day and 6% of patients receiving Savella 200 mg/day. Sustained increases in DBP (increase of ≥ 10 mmHg on 3 consecutive post-baseline visits) occurred in 4% of patients receiving placebo versus 13% of patients receiving Savella 100 mg/day and 10% of patients receiving Savella 200 mg/day.
Sustained increases in blood pressure could have adverse consequences. Cases of elevated blood pressure requiring immediate treatment have been reported.
Concomitant use of Savella with drugs that increase blood pressure and pulse has not been evaluated and such combinations should be used with caution [see Drug Interactions (7)].
Effects of Savella on blood pressure in patients with significant hypertension or cardiac disease have not been systematically evaluated. Savella should be used with caution in these patients.
Blood pressure should be measured prior to initiating treatment and periodically measured throughout Savella treatment. Pre-existing hypertension and other cardiovascular disease should be treated before starting therapy with Savella. For patients who experience a sustained increase in blood pressure while receiving Savella, either dose reduction or discontinuation should be considered.
5.4 Effects on Heart Rate
SNRIs have been associated with reports of increase in heart rate.
In clinical trials, relative to placebo, Savella treatment was associated with mean increases in pulse rate of approximately 7 to 8 beats per minute [see Adverse Reactions (6.2,6.3)].
Increases in pulse ≥ 20 bpm occurred more frequently in Savella-treated patients when compared to placebo: 0.3% in the placebo arm versus 8% in the Savella 100 mg/day and 8% in the 200 mg/day treatment arms. The effect of Savella on heart rate did not appear to increase with increasing dose.
Savella has not been systematically evaluated in patients with a cardiac rhythm disorder.
Heart rate should be measured prior to initiating treatment and periodically measured throughout Savella treatment. Pre-existing tachyarrhythmias and other cardiac disease should be treated before starting therapy with Savella. For patients who experience a sustained increase in heart rate while receiving Savella, either dose reduction or discontinuation should be considered.
5.5 Seizures
Savella has not been systematically evaluated in patients with a seizure disorder. In clinical trials evaluating Savella in patients with fibromyalgia, seizures/convulsions have not been reported. However, seizures have been reported infrequently in patients treated with Savella for disorders other than fibromyalgia. Savella should be prescribed with care in patients with a history of a seizure disorder.
5.6 Hepatotoxicity
In the placebo-controlled fibromyalgia trials, increases in the number of patients treated with Savella with mild elevations of ALT or AST (1-3 times the upper limit of normal, ULN) were observed. Increases in ALT were more frequently observed in the patients treated with Savella 100 mg/day (6%) and Savella 200 mg/day (7%), compared to the patients treated with placebo (3%). One patient receiving Savella 100 mg/day (0.2%) had an increase in ALT greater than 5 times the upper limit of normal but did not exceed 10 times the upper limit of normal. Increases in AST were more frequently observed in the patients treated with Savella 100 mg/day (3%) and Savella 200 mg/day (5%) compared to the patients treated with placebo (2%).
The increases of bilirubin observed in the fibromyalgia clinical trials were not clinically significant.
No case met the criteria of elevated ALT > 3x ULN and associated with an increase in bilirubin ≥ 2x ULN.
There have been cases of increased liver enzymes and reports of severe liver injury, including fulminant hepatitis with milnacipran from foreign postmarketing experience. In the cases of severe liver injury there were significant underlying clinical conditions and/or the use of multiple concomitant medications. Because of underreporting, it is impossible to provide an accurate estimate of the true incidence of these reactions.
Savella should be discontinued in patients who develop jaundice or other evidence of liver dysfunction. Treatment with Savella should not be resumed unless another cause can be established.
Savella should ordinarily not be prescribed to patients with substantial alcohol use or evidence of chronic liver disease.
5.7 Discontinuation of Treatment with Savella
Withdrawal symptoms have been observed in clinical trials following discontinuation of milnacipran, as with other SNRIs and SSRIs.
During marketing of milnacipran, and other SNRIs and SSRIs, there have been spontaneous reports of adverse events indicative of withdrawal and physical dependence occurring upon discontinuation of these drugs, particularly when discontinuation is abrupt. The adverse events include the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. Although these events are generally self-limiting, some have been reported to be severe.
Patients should be monitored for these symptoms when discontinuing treatment with Savella. Savella should be tapered and not abruptly discontinued after extended use. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate [see Dosage and Administration (2.4)].
5.8 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including Savella. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SNRIs, SSRIs, or Savella. Also, patients taking diuretics or who are otherwise volume-depleted may be at greater risk [see Geriatric Use (8.5)]. Discontinuation of Savella should be considered in patients with symptomatic hyponatremia.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.9 Abnormal Bleeding
SSRIs and SNRIs, including Savella, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs, warfarin, and other anti-coagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs and SNRIs use have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Patients should be cautioned about the risk of bleeding associated with the concomitant use of Savella and NSAIDs, aspirin, or other drugs that affect coagulation.
5.10 Activation of Mania
No activation of mania or hypomania was reported in the clinical trials evaluating effects of Savella in patients with fibromyalgia. However those clinical trials excluded patients with current major depressive episode. Activation of mania and hypomania have been reported in patients with mood disorders who were treated with other similar drugs for major depressive disorder. As with these other agents, Savella should be used cautiously in patients with a history of mania.
5.11 Patients with a History of Dysuria
Because of their noradrenergic effect, SNRIs including Savella, can affect urethral resistance and micturition. In the controlled fibromyalgia trials, dysuria occurred more frequently in patients treated with Savella (1%) than in placebo-treated patients (0.5%). Caution is advised in use of Savella in patients with a history of dysuria, notably in male patients with prostatic hypertrophy, prostatitis, and other lower urinary tract obstructive disorders. Male patients are more prone to genitourinary adverse effects, such as dysuria or urinary retention, and may experience testicular pain or ejaculation disorders.
5.12 Controlled Narrow-Angle Glaucoma
Mydriasis has been reported in association with SNRIs and Savella; therefore, Savella should be used cautiously in patients with controlled narrow-angle glaucoma.
Do not use Savella in patients with Uncontrolled Narrow-Angle Glaucoma [see Contraindications (4.2)].
5.13 Concomitant Use with Alcohol
In clinical trials, more patients treated with Savella developed elevated transaminases than did placebo treated patients [see Warnings and Precautions (5.6)]. Because it is possible that milnacipran may aggravate pre-existing liver disease, Savella should not be prescribed to patients with substantial alcohol use or evidence of chronic liver disease.
5.14 Allergy to FD&C Yellow No. 5
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
6 ADVERSE REACTIONS
6.1 Clinical Trial Data Sources
Savella was evaluated in three double-blind placebo-controlled trials involving 2209 fibromyalgia patients (1557 patients treated with Savella and 652 patients treated with placebo) for a treatment period up to 29 weeks.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse reaction of the type listed. A reaction was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Adverse Reactions Leading to Discontinuation
In placebo-controlled trials in patients with fibromyalgia, 23% of patients treated with Savella 100 mg/day, 26% of patients treated with Savella 200 mg/day discontinued prematurely due to adverse reactions, compared to 12% of patients treated with placebo. The adverse reactions that led to withdrawal in ≥ 1% of patients in the Savella treatment group and with an incidence rate greater than that in the placebo treatment group were nausea (milnacipran 6%, placebo 1%), palpitations (milnacipran 3%, placebo 1%), headache (milnacipran 2%, placebo 0%), constipation (milnacipran 1%, placebo 0%), heart rate increased (milnacipran 1%, placebo 0%), and hyperhidrosis (milnacipran 1%, placebo 0%), vomiting (milnacipran 1%, placebo 0%), and dizziness (milnacipran 1% and placebo 0.5%). Discontinuation due to adverse reactions was generally more common among patients treated with Savella 200 mg/day compared to Savella 100 mg/day.
6.3 Most Common Adverse Reactions
In the placebo-controlled fibromyalgia patient trials the most frequently occurring adverse reaction in clinical trials was nausea. The most common adverse reactions (incidence ≥ 5% and twice placebo) in patients treated with Savella were constipation, hot flush, hyperhidrosis, vomiting, palpitations, heart rate increased, dry mouth, and hypertension.
Table 2 lists all adverse reactions that occurred in at least 2% of patients treated with Savella at either 100 or 200 mg/day and at an incidence greater than that of placebo.
| System Organ Class–
Preferred Term | Savella
100 mg/day (n = 623) % | Savella
200 mg/day (n = 934) % | All Savella
(n = 1557) % | Placebo
(n = 652) % |
| Cardiac Disorders | ||||
| Palpitations | 8 | 7 | 7 | 2 |
| Tachycardia | 3 | 2 | 2 | 1 |
| Eye Disorders | ||||
| Vision blurred | 1 | 2 | 2 | 1 |
| Gastrointestinal Disorders | ||||
| Nausea | 35 | 39 | 37 | 20 |
| Constipation | 16 | 15 | 16 | 4 |
| Vomiting | 6 | 7 | 7 | 2 |
| Dry mouth | 5 | 5 | 5 | 2 |
| Abdominal pain | 3 | 3 | 3 | 2 |
| General Disorders | ||||
| Chest pain | 3 | 2 | 2 | 2 |
| Chills | 1 | 2 | 2 | 0 |
| Chest discomfort | 2 | 1 | 1 | 1 |
| Infections | ||||
| Upper respiratory tract infection | 7 | 6 | 6 | 6 |
| Investigations | ||||
| Heart rate increased | 5 | 6 | 6 | 1 |
| Blood pressure increased | 3 | 3 | 3 | 1 |
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite | 1 | 2 | 2 | 0 |
| Nervous System Disorders | ||||
| Headache | 19 | 17 | 18 | 14 |
| Dizziness | 11 | 10 | 10 | 6 |
| Migraine | 6 | 4 | 5 | 3 |
| Paresthesia | 2 | 3 | 2 | 2 |
| Tremor | 2 | 2 | 2 | 1 |
| Hypoesthesia | 1 | 2 | 1 | 1 |
| Tension headache | 2 | 1 | 1 | 1 |
| Psychiatric Disorders | ||||
| Insomnia | 12 | 12 | 12 | 10 |
| Anxiety | 5 | 3 | 4 | 4 |
| Respiratory Disorders | ||||
| Dyspnea | 2 | 2 | 2 | 1 |
| Skin Disorders | ||||
| Hyperhidrosis | 8 | 9 | 9 | 2 |
| Rash | 3 | 4 | 3 | 2 |
| Pruritus | 3 | 2 | 2 | 2 |
| Vascular Disorders | ||||
| Hot flush | 11 | 12 | 12 | 2 |
| Hypertension | 7 | 4 | 5 | 2 |
| Flushing | 2 | 3 | 3 | 1 |
6.4 Weight Changes
In placebo-controlled fibromyalgia clinical trials, patients treated with Savella for up to 3 months experienced a mean weight loss of approximately 0.8 kg in both the Savella 100 mg/day and the Savella 200 mg/day treatment groups, compared with a mean weight loss of approximately 0.2 kg in placebo-treated patients.
6.5 Genitourinary Adverse Reactions in Males
In the placebo-controlled fibromyalgia studies, the following treatment-emergent adverse reactions related to the genitourinary system were observed in at least 2% of male patients treated with Savella, and occurred at a rate greater than in placebo-treated male patients: dysuria, ejaculation disorder, erectile dysfunction, ejaculation failure, libido decreased, prostatitis, scrotal pain, testicular pain, testicular swelling, urinary hesitation, urinary retention, urethral pain, and urine flow decreased.
6.6 Other Adverse Reactions Observed During Clinical Trials of Savella in Fibromyalgia
Following is a list of frequent (those occurring on one or more occasions in at least 1/100 patients) treatment-emergent adverse reactions reported from 1824 fibromyalgia patients treated with Savella for periods up to 68 weeks. The listing does not include those events already listed in Table 1, those events for which a drug cause was remote, those events which were so general as to be uninformative, and those events reported only once which did not have a substantial probability of being acutely life threatening.
Adverse reactions are categorized by body system and listed in order of decreasing frequency. Adverse reactions of major clinical importance are described in the Warnings and Precautions section (5).
Gastrointestinal Disorders - diarrhea, dyspepsia, gastroesophageal reflux disease, flatulence, abdominal distension
General Disorders - fatigue, peripheral edema, irritability, pyrexia
Infections - urinary tract infection, cystitis
Injury, Poisoning, and Procedural Complications - contusion, fall
Investigations - weight decreased or increased
Metabolism and Nutrition Disorders - hypercholesterolemia
Nervous System Disorders - somnolence, dysgeusia
Psychiatric Disorders - depression, stress
Skin Disorders - night sweats
6.7 Postmarketing Spontaneous Reports
The following additional adverse reactions have been identified from spontaneous reports of Savella received worldwide. These adverse reactions have been chosen for inclusion because of a combination of seriousness, frequency of reporting, or potential causal connection to Savella. However, because these adverse reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events include:
Blood and Lymphatic System Disorders - leukopenia, neutropenia, thrombocytopenia
Cardiac Disorders - supraventricular tachycardia
Eye Disorders - accommodation disorder
Endocrine Disorders - hyperprolactinemia
Hepatobiliary Disorders - hepatitis
Metabolism and Nutrition Disorders - anorexia, hyponatremia
Musculoskeletal and Connective Tissue Disorders - rhabdomyolysis
Nervous System Disorders - convulsions (including grand mal), loss of consciousness, neuroleptic malignant syndrome, Parkinsonism, serotonin syndrome
Psychiatric Disorders - delirium, hallucination
Renal and Urinary Disorders - acute renal failure
Reproductive System and Breast Disorders - galactorrhea
Skin Disorders - erythema multiforme, Stevens Johnson syndrome
Vascular Disorders - hypertensive crisis
7 DRUG INTERACTIONS
Milnacipran undergoes minimal CYP450 related metabolism, with the majority of the dose excreted unchanged in urine (55%), and has a low binding to plasma proteins (13%). In vitro and in vivo studies showed that Savella is unlikely to be involved in clinically significant pharmacokinetic drug interactions [see Pharmacokinetics in Special Populations (12.4)].
7.2 Serotonergic Drugs
Due to the mechanism of action of SNRIs and SSRIs, including Savella, and the potential for serotonin syndrome or Neuroleptic Malignant Syndrome (NMS) – like reactions, caution is advised when Savella is co-administered with other drugs that may affect the serotonergic neurotransmitter systems. This includes drugs such as triptans, lithium, tryptophan, antipsychotics and dopamine antagonists.
Co-administration of Savella with other inhibitors of serotonin re-uptake may result in hypertension and coronary artery vasoconstriction, through additive serotonergic effects. Concomitant use of Savella with other SSRIs, SNRIs, or tryptophan is not recommended [see Warnings and Precautions (5.2)].
7.3 Triptans
There have been rare postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of Savella with a triptan is clinically warranted, careful observation of the patient is advised, , particularly during treatment initiation and dose increases. [see Warnings and Precautions (5.2)]
7.4 Catecholamines
Savella inhibits the reuptake of norepinephrine. Therefore concomitant use of Savella with epinephrine and norepinephrine may be associated with paroxysmal hypertension and possible arrhythmia [see Warnings and Precautions – Effects on Blood Pressure (5.3) and Effects on Heart Rate (5.4)]
7.5 CNS-active drugs
Given the primary CNS effects of Savella, caution should be used when it is taken in combination with other centrally acting drugs, including those with a similar mechanism of action.
7.6 Clinically Important Interactions with Select Cardiovascular Agents
Digoxin: Use of Savella concomitantly with digoxin may be associated with potentiation of adverse hemodynamic effects. Postural hypotension and tachycardia have been reported in combination therapy with intravenously administered digoxin (1 mg). Co-administration of Savella and intravenous digoxin should be avoided [see Warnings and Precautions (5.3, 5.4)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Milnacipran increased the incidence of dead fetuses in utero in rats at doses of 5 mg/kg/day (0.25 times the MRHD on a mg/m2 basis). Administration of milnacipran to mice and rabbits during the period of organogenesis did not result in embryotoxicity or teratogenicity at doses up to 125 mg/kg/day in mice (3 times the maximum recommended human dose [MRHD] of 200 mg/day on a mg/m2 basis) and up to 60 mg/kg/day in rabbits (6 times the MRHD of 200 mg/day on a mg m2 basis). In rabbits, the incidence of the skeletal variation, extra single rib, was increased following administration of milnacipran at 15 mg/kg/day during the period of organogenesis.
There are no adequate and well-controlled studies in pregnant women. Savella should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
To provide information regarding the exposure to Savella during pregnancy, Physicians are advised to recommend that pregnant patients taking Savella enroll in the Savella Pregnancy Registry. Enrollment is voluntary and may be initiated by pregnant patients or their healthcare providers by contacting the registry at 1-877-643-3010 or by email at registries@kendle.com. Data forms may also be downloaded from the registry website at www.savellapregnancyregistry.com.
Nonteratogenic Effects
Neonates exposed to dual reuptake inhibitors of serotonin and norepinephrine, or selective serotonin reuptake inhibitors late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of these classes of drugs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.2)].
In rats, a decrease in pup body weight and viability on postpartum day 4 were observed when milnacipran, at a dose of 5 mg/kg/day (approximately 0.2 times the MRHD on a mg/m2 basis), was administered orally to rats during late gestation. The no-effect dose for maternal and offspring toxicity was 2.5 mg/kg/day (approximately 0.1 times the MRHD on a mg/m2 basis).
8.2 Labor and Delivery
The effect of milnacipran on labor and delivery is unknown. The use of Savella during labor and delivery is not recommended.
8.3 Nursing Mothers
There are no adequate and well-controlled studies in nursing mothers. It is not known if milnacipran is excreted in human milk. Studies in animals have shown that milnacipran or its metabolites are excreted in breast milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from milnacipran, a decision should be made whether to discontinue the drug, taking into account the importance of the drug to the mother. Because the safety of Savella in infants is not known, nursing while on Savella is not recommended.
8.4 Pediatric Use
Safety and effectiveness of Savella in a fibromyalgia pediatric population below the age of 17 have not been established [see Box Warning and Warnings and Precautions (5.1)]. The use of Savella is not recommended in pediatric patients.
8.5 Geriatric Use
In controlled clinical studies of Savella, 402 patients were 60 years or older, and no overall differences in safety and efficacy were observed between these patients and younger patients.
In view of the predominant excretion of unchanged milnacipran via kidneys and the expected decrease in renal function with age renal function should be considered prior to use of Savella in the elderly [see Dosage and Administration (2.2)].
SNRIs, SSRIs, and Savella, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event [see Warnings and Precautions (5.8)].
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Milnacipran did not produce behavioral signs indicative of abuse potential in animal or human studies.
9.3 Dependence
Milnacipran produces physical dependence, as evidenced by the emergence of withdrawal symptoms following drug discontinuation, similar to other SNRIs and SSRIs. These withdrawal symptoms can be severe. Thus, Savella should be tapered and not abruptly discontinued after extended use. [see Section 5.7 Discontinuation of Treatment with Savella].
10 OVERDOSAGE
There is limited clinical experience with Savella overdose in humans. In clinical trials, cases of acute ingestions up to 1000 mg, alone or in combination with other drugs, were reported with none being fatal.
In postmarketing experience, fatal outcomes have been reported for acute overdoses primarily involving multiple drugs but also with Savella only. The most common signs and symptoms included increased blood pressure, cardio-respiratory arrest, changes in the level of consciousness (ranging from somnolence to coma), confusional state, dizziness, and increased hepatic enzymes.
Management of Overdose
There is no specific antidote to Savella, but if serotonin syndrome ensues, specific treatment (such as with cyproheptadine and/or temperature control) may be considered. In case of acute overdose, treatment should consist of those general measures employed in the management of overdose with any drug.
An adequate airway, oxygenation, and ventilation should be assured and cardiac rhythm and vital signs should be monitored. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients. Because there is no specific antidote for Savella, symptomatic care and treatment with gastric lavage and activated charcoal should be considered as soon as possible for patients who experience a Savella overdose.
Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be beneficial.
In managing overdose, the possibility of multiple drug involvement should be considered. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians' Desk Reference (PDR).
11 DESCRIPTION
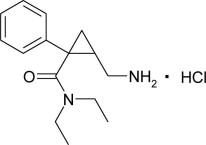
Milnacipran hydrochloride is a selective norepinephrine and serotonin reuptake inhibitor; it inhibits norepinephrine uptake with greater potency than serotonin. It is a racemic mixture with the chemical name: (±)-[1R(S),2S(R)]-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride. The structural formula is:
Milnacipran hydrochloride is a white to off-white crystalline powder with a melting point of 179°C.
It is freely soluble in water, methanol, ethanol, chloroform, and methylene chloride and sparingly soluble in diethyl ether. It has an empirical formula of C15H23ClN2O and a molecular weight of 282.8 g/mol.
Savella is available for oral administration as film-coated tablets containing 12.5 mg, 25 mg, 50 mg, and 100 mg milnacipran hydrochloride. Each tablet also contains dibasic calcium phosphate, povidone, carboxymethylcellulose calcium, colloidal silicon dioxide, magnesium stearate, and talc as inactive ingredients. Additionally, the following inactive ingredients are also present as components of the film coat:
12.5 mg:
FD&C Red #40 Aluminum Lake dye, hypromellose, polyethylene glycol, titanium dioxide
25 mg:
Hypromellose, polyethylene glycol, titanium dioxide
50 mg:
FD&C Blue #1, Blue #2, and Yellow #5 Aluminum Lake dyes; hypromellose; polyethylene glycol; titanium dioxide
100 mg:
FD&C Blue #1 Aluminum Lake dye, hypromellose, polyethylene glycol, titanium dioxide
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of the central pain inhibitory action of milnacipran and its ability to improve the symptoms of fibromyalgia in humans are unknown. Preclinical studies have shown that milnacipran is a potent inhibitor of neuronal norepinephrine and serotonin reuptake; milnacipran inhibits norepinephrine uptake with approximately 3-fold higher potency in vitro than serotonin without directly affecting the uptake of dopamine or other neurotransmitters. Milnacipran has no significant affinity for serotonergic (5-HT1-7), α- and β-adrenergic, muscarinic (M1-5), histamine (H1-4), dopamine (D1-5), opiate, benzodiazepine, and γ-aminobutyric acid (GABA) receptors in vitro. Pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Milnacipran has no significant affinity for Ca++, K+, Na+ and Cl– channels and does not inhibit the activity of human monoamine oxidases (MAO-A and MAO-B) or acetylcholinesterase.
12.2 Pharmacodynamics
Cardiovascular Electrophysiology-The effect of Savella on the QTcF interval was measured in a double-blind placebo- and positive-controlled parallel study in 88 healthy subjects using 600 mg/day Savella (3 to 6 times the recommended therapeutic dose for fibromyalgia). After baseline and placebo adjustment, the maximum mean QTcF change was 8 ms (2-sided 90% CI, 3 - 12 ms). This increase is not considered to be clinically significant.
12.3 Pharmacokinetics
Milnacipran is well absorbed after oral administration with an absolute bioavailability of approximately 85% to 90%. The exposure to milnacipran increased proportionally within the therapeutic dose range. It is excreted predominantly unchanged in urine (55%) and has a terminal elimination half-life of about 6 to 8 hours. Steady-state levels are reached within 36 to 48 hours and can be predicted from single-dose data. The active enantiomer, d-milnacipran, has a longer elimination half-life (8-10 hours) than the l-enantiomer (4-6 hours). There is no interconversion between the enantiomers.
Absorption and Distribution
Savella is absorbed following oral administration with maximum concentrations (Cmax) reached within 2 to 4 hours post dose. Absorption of Savella is not affected by food. The absolute bioavailability is approximately 85% to 90%. The mean volume of distribution of milnacipran following a single intravenous dose to healthy subjects is approximately 400 L.
Plasma protein binding is 13%.
Metabolism and Elimination
Milnacipran and its metabolites are eliminated primarily by renal excretion. Following oral administration of 14C-milnacipran hydrochloride, approximately 55% of the dose was excreted in urine as unchanged milnacipran (24% as l-milnacipran and 31% as d-milnacipran). The l-milnacipran carbamoyl-O-glucuronide was the major metabolite excreted in urine and accounted for approximately 17% of the dose; approximately 2% of the dose was excreted in urine as d-milnacipran carbamoyl-O-glucuronide. Approximately 8% of the dose was excreted in urine as the N-desethyl milnacipran metabolite.
12.4 Pharmacokinetics in Special Populations
Renal Impairment-Milnacipran pharmacokinetics were evaluated following single oral administration of 50 mg Savella to subjects with mild (creatinine clearance [CLcr] 50-80 mL/min), moderate (CLcr 30-49 mL/min), and severe (CLcr 5-29 mL/min) renal impairment and to healthy subjects (CLcr > 80 mL/min). The mean AUC0-∞ increased by 16%, 52%, and 199%, and terminal elimination half-life increased by 38%, 41%, and 122% in subjects with mild, moderate, and severe renal impairment, respectively, compared with healthy subjects.
No dosage adjustment is necessary for patients with mild renal impairment. Caution should be exercised in patients with moderate renal impairment. Dose adjustment is necessary in severe renal impairment patients. [see Dosage and Administration (2.2)].
Hepatic Impairment-Milnacipran pharmacokinetics were evaluated following single oral administration of 50 mg Savella to subjects with mild (Child-Pugh A), moderate (Child-Pugh B), and severe (Child-Pugh C) hepatic impairment and to healthy subjects. AUC0-∞ and T½ were similar in healthy subjects and subjects with mild and moderate hepatic impairment. However, subjects with severe hepatic impairment had a 31% higher AUC0-∞ and a 55% higher T½ than healthy subjects. Caution should be exercised in patients with severe hepatic impairment.
Elderly-Cmax and AUC parameters of milnacipran were about 30% higher in elderly (> 65 years) subjects compared with young subjects due to age-related decreases in renal function. No dosage adjustment is necessary based on age unless renal function is severely impaired [see Dosage and Administration (2.2)].
Gender-Cmax and AUC parameters of milnacipran were about 20% higher in female subjects compared with male subjects. Dosage adjustment based on gender is not necessary.
In Vitro Studies
In general, milnacipran, at concentrations that were at least 25 times those attained in clinical trials, did not inhibit human CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 or induce human CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A4/5 enzyme systems, indicating a low potential of interactions with drugs metabolized by these enzymes.
In vitro studies have shown that the biotransformation rate of milnacipran by human hepatic microsomes and hepatocytes was low. A low biotransformation was also observed following incubation of milnacipran with cDNA-expressed human CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 isozymes.
In Vivo Studies
The drug interaction studies described in this section were conducted in healthy adult subjects.
Carbamazepine-There were no clinically significant changes in the pharmacokinetics of milnacipran following coadministration of Savella (100 mg/day) and carbamazepine (200 mg twice a day). No changes were observed in the pharmacokinetics of carbamazepine or its epoxide metabolite due to coadministration with Savella.
Clomipramine-Switch from clomipramine (75 mg once a day) to milnacipran (100 mg/day) without a washout period did not lead to clinically significant changes in the pharmacokinetics of milnacipran. Because an increase in adverse events (eg, euphoria and postural hypotension) was observed after switching from clomipramine to milnacipran, monitoring of patients during treatment switch is recommended.
Digoxin-There was no pharmacokinetic interaction between Savella (200 mg/day) and digoxin (0.2 mg/day Lanoxicaps) following multiple-dose administration to healthy subjects.
Fluoxetine-Switch from fluoxetine (20 mg once a day), a strong inhibitor of CYP2D6 and a moderate inhibitor of CYP2C19, to milnacipran (100 mg/day) without a washout period did not affect the pharmacokinetics of milnacipran.
Lithium-Multiple doses of Savella (100 mg/day) did not affect the pharmacokinetics of lithium.
Lorazepam-There was no pharmacokinetic interaction between a single dose of Savella (50 mg) and lorazepam (1.5 mg).
Warfarin-Steady-state milnacipran (200 mg/day) did not affect the pharmacokinetics of R-warfarin and S-warfarin or the pharmacodynamics (as assessed by measurement of prothrombin INR) of a single dose of 25 mg warfarin. The pharmacokinetics of Savella were not altered by warfarin.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Dietary administration of milnacipran to rats at doses of 50 mg/kg/day (2 times the MRHD on a mg/m2 basis) for 2 years caused a statistically significant increase in the incidence of thyroid C-cell adenomas and combined adenomas and carcinomas in males. A carcinogenicity study was conducted in Tg.rasH2 mice for 6 months at oral gavage doses of up to 125 mg/kg/day.
Milnacipran did not induce tumors in Tg.rasH2 mice at any dose tested.
Mutagenesis
Milnacipran was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test) or in the L5178Y TK +/- mouse lymphoma forward mutation assay. Milnacipran was also not clastogenic in an in vitro chromosomal aberration test in human lymphocytes or in the in vivo mouse micronucleus assay.
Impairment of Fertility
Although administration of milnacipran to male and female rats had no statistically significant effect on mating or fertility at doses up to 80 mg/kg/day (4 times the MRHD on an mg/m2 basis) there was an apparent dose-related decrease in the fertility index at clinically relevant doses based on body surface area.
13.2 Animal Toxicology and/or Pharmacology
Hepatic Effects
Chronic administration (2-years) of milnacipran to rats at 15 mg/kg (0.6 times the MRHD on an mg/m2 basis) and higher doses showed increased incidences of centrilobular vacuolation of the liver in male rats and eosinophilic foci in male and female rats in the absence of any change in hepatic enzymes. The clinical significance of the finding is not known. Chronic (1-year) administration in the primate at doses up to 25 mg/kg (2 times the MRHD on a mg/m2 basis) did not demonstrate similar evidence of hepatic changes.
14 CLINICAL STUDIES
Management of Fibromyalgia
The efficacy of Savella for the management of fibromyalgia was established in two double-blind, placebo-controlled, multicenter studies in adult patients (18-74 years of age). Enrolled patients met the American College of Rheumatology (ACR) criteria for fibromyalgia (a history of widespread pain for 3 months and pain present at 11 or more of the 18 specific tender point sites). Approximately 35% of patients had a history of depression. Study 1 was six months in duration and Study 2 was three months in duration.
A larger proportion of patients treated with Savella than with placebo experienced a simultaneous reduction in pain from baseline of at least 30% (VAS) and also rated themselves as much improved or very much improved based on the patient global assessment (PGIC). In addition, a larger proportion of patients treated with Savella met the criteria for treatment response, as measured by the composite endpoint that concurrently evaluated improvement in pain (VAS), physical function (SF-36 PCS), and patient global assessment (PGIC), in fibromyalgia as compared to placebo.
Study 1: This 6-month study compared total daily doses of Savella 100 mg and 200 mg to placebo. Patients were enrolled with a minimum mean baseline pain score of ≥ 50 mm on a 100 mm visual analog scale (VAS) ranging from 0 (“no pain”) to 100 (“worst possible pain”). The mean baseline pain score in this trial was 69. The efficacy results for Study 1 are summarized in Figure 1.
Figure 1 shows the proportion of patients achieving various degrees of improvement in pain from baseline to the 3-month time point and who concurrently rated themselves globally improved (PGIC score of 1 or 2). Patients who did not complete the 3-month assessment were assigned 0% improvement. More patients in the Savella treatment arms experienced at least a 30% reduction in pain from baseline (VAS) and considered themselves globally improved (PGIC) than did patients in the placebo arm. Treatment with Savella 200 mg/day did not confer greater benefit than treatment with Savella 100 mg/day.
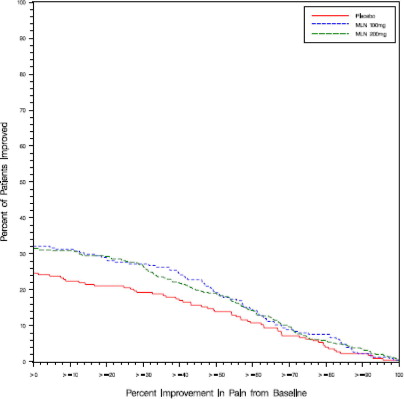
Figure 1: Patients Achieving Various Levels of Pain Relief with Concurrent Ratings of Being Much or Very Much Improved on the PGIC - Study 1
Study 2: This 3-month study compared total daily doses of Savella 100 mg and 200 mg to placebo. Patients were enrolled with a minimum mean baseline pain score of ≥ 40 mm on a 100- mm VAS ranging from 0 (“no pain”) to 100 (“worst possible pain”). The mean baseline pain score in this trial was 65. The efficacy results for Study 2 are summarized in Figure 2.
Figure 2 shows the proportion of patients achieving various degrees of improvement in pain from baseline to the 3-month time point and who concurrently rated themselves globally improved (PGIC score of 1 or 2). Patients who did not complete the 3-month assessment were assigned 0% improvement. More patients in the Savella treatment arms experienced at least a 30% reduction in pain from baseline (VAS) and considered themselves globally improved (PGIC) than did patients in the placebo arm. Treatment with Savella 200 mg/day did not confer greater benefit than treatment with Savella 100 mg/day.
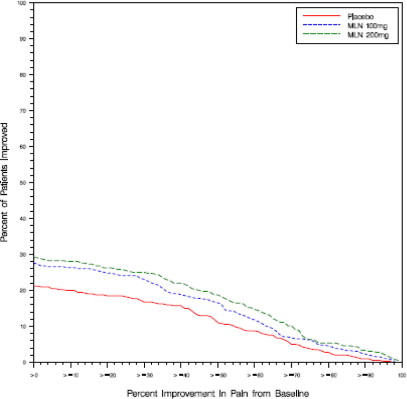
Figure 2: Patients Achieving Various Levels of Pain Relief with Concurrent Ratings of Being Much or Very Much Improved on the PGIC - Study 2
In both studies, some patients who rated themselves as globally “much” or “very much” improved experienced a decrease in pain as early as week 1 of treatment with a stable dose of Savella that persisted throughout these studies.
16 HOW SUPPLIED/STORAGE AND HANDLING
25-mg tablets:
White, round, film-coated tablets, debossed with “FL” on one side and “25” on the reverse
Bottles of 60: NDC 0456-1525-60
50-mg tablets:
White, oval-shaped, film-coated tablets, debossed with “FL” on one side and “50” on the reverse
Bottles of 60: NDC 0456-1550-60
Storage
Store at 25°C (77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F) [See USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See Medication Guide
17.1 Information in Medication Guide
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Savella and should counsel them in its appropriate use. A patient Medication Guide is available for Savella. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Savella:
17.2 Suicide Risk
Patients and their families and caregivers should be advised that Savella is a selective norepinephrine and serotonin reuptake inhibitor and therefore belongs to the same class of drugs as antidepressants. Patients, their families and their caregivers should be advised that patients with depression may be at increased risk for clinical worsening and/or suicidal ideation if they stop taking anti-depressant medication, change the dose, or start a new medication.
Patients, their families and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania or other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during treatment with Savella or other drugs that inhibit the reuptake of norepinephrine and/or serotonin, and when the dose is adjusted up or down. Families and caregivers of patients should be advised to observe for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. [see Box Warning and Warnings and Precautions (5.1)].
17.3 Serotonin Syndrome
Patients should be cautioned about the risk of serotonin syndrome with concomitant use of Savella and triptans, tramadol, or other serotonergic agents [see Warnings and Precautions (5.2)].
17.4 Effect on Blood Pressure and Pulse
Patients should be advised that their blood pressure and pulse should be monitored at regular intervals when receiving treatment with Savella [see Warnings and Precautions (5.3, 5.4)].
17.5 Abnormal Bleeding
Patients should be cautioned about the concomitant use of Savella and NSAIDs, aspirin, or other drugs that affect coagulation, since the combined use of agents that interfere with serotonin reuptake and these agents has been associated with an increased risk of abnormal bleeding [see Warnings and Precautions (5.9)].
17.6 Ability to Drive and Use Machinery
Savella might diminish mental and physical capacities necessary to perform certain tasks such as operating machinery, including motor vehicles. Patients should be cautioned about operating machinery or driving motor vehicles until they are reasonably certain that Savella treatment does not affect their ability to engage in such activities.
17.7 Alcohol
Patients should be advised to avoid consumption of alcohol while taking Savella [see Warnings and Precautions (5.6, 5.13)].
17.8 Discontinuation
Patients should be advised that withdrawal symptoms can occur when discontinuing treatment with Savella, particularly when discontinuation is abrupt. [see Warnings and Precautions (5.7)]
17.9 Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during Savella therapy [see Use in Specific Populations (8.1)].
Patients should be encouraged to enroll in the Savella Pregnancy Registry if they become pregnant, preferably before any prenatal testing is done. This registry is collecting information about the safety of milnacipran during pregnancy. To enroll, patients or their healthcare providers may call the toll free number 1-877-643-3010 [see Use in Specific Populations (8.1)], download data forms from our website, www.savellapregnancyregistry.com, or email the registry for further information at registries@kendle.com.
17.10 Nursing
Patients should be advised to notify their physician if they are breast-feeding [see Use in Specific Populations (8.3)].
MEDICATION GUIDE
Savella® (Sa-vel-la) Tablets
(milnacipran HCl)
Antidepressant Medicines, Depression and other serious Mental Illnesses, and Suicidal
Thoughts or Actions
Savella is not used to treat depression, but it acts like medicines that are used to treat depression (antidepressants) and other psychiatric disorders.
Read the Medication Guide that comes with you or your family member's antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts or actions with antidepressant medicines. Talk to your or your family member's healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manicdepressive illness) or suicidal thoughts or actions.
-
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
|
|
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA- 1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: January 2009
Manufactured for:
Forest Pharmaceuticals, Inc.
Manufactured by:
Forest Laboratories, Inc.
Licensed from Pierre Fabre Medicament and Cypress Bioscience, Inc.
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320