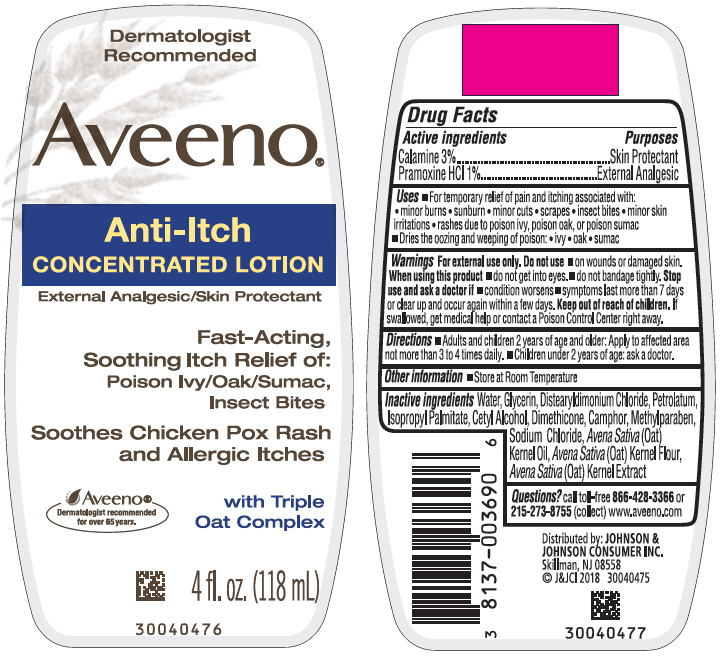
Uses
- For temporary relief of pain and itching associated with:
- minor burns
- sunburn
- minor cuts
- scrapes
- insect bites
- minor skin irritations
- rashes due to poison ivy, poison oak, or poison sumac
- Dries the oozing and weeping of poison:
- ivy
- oak
- sumac
Warnings
For external use only.
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: ask a doctor.
Inactive ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Dimethicone, Camphor, Methylparaben, Sodium Chloride, Avena Sativa (Oat) Kernel Oil, Avena Sativa (Oat) Kernal Flour, Avena Sativa (Oat) Kernel Extract
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
Dermatologist
Recommended
Aveeno ®
Anti-Itch
CONCENTRATED LOTION
External Analgesic/Skin Protectant
Fast-Acting,
Soothing Itch Relief of:
Poison Ivy/Oak/Sumac,
Insect Bites
Soothes Chicken Pox Rash
and Allergic Itches
Aveeno
®
Dermatologist recommended
for over 65 years
with Triple
Oat Complex
4 fl. oz. (118 mL)