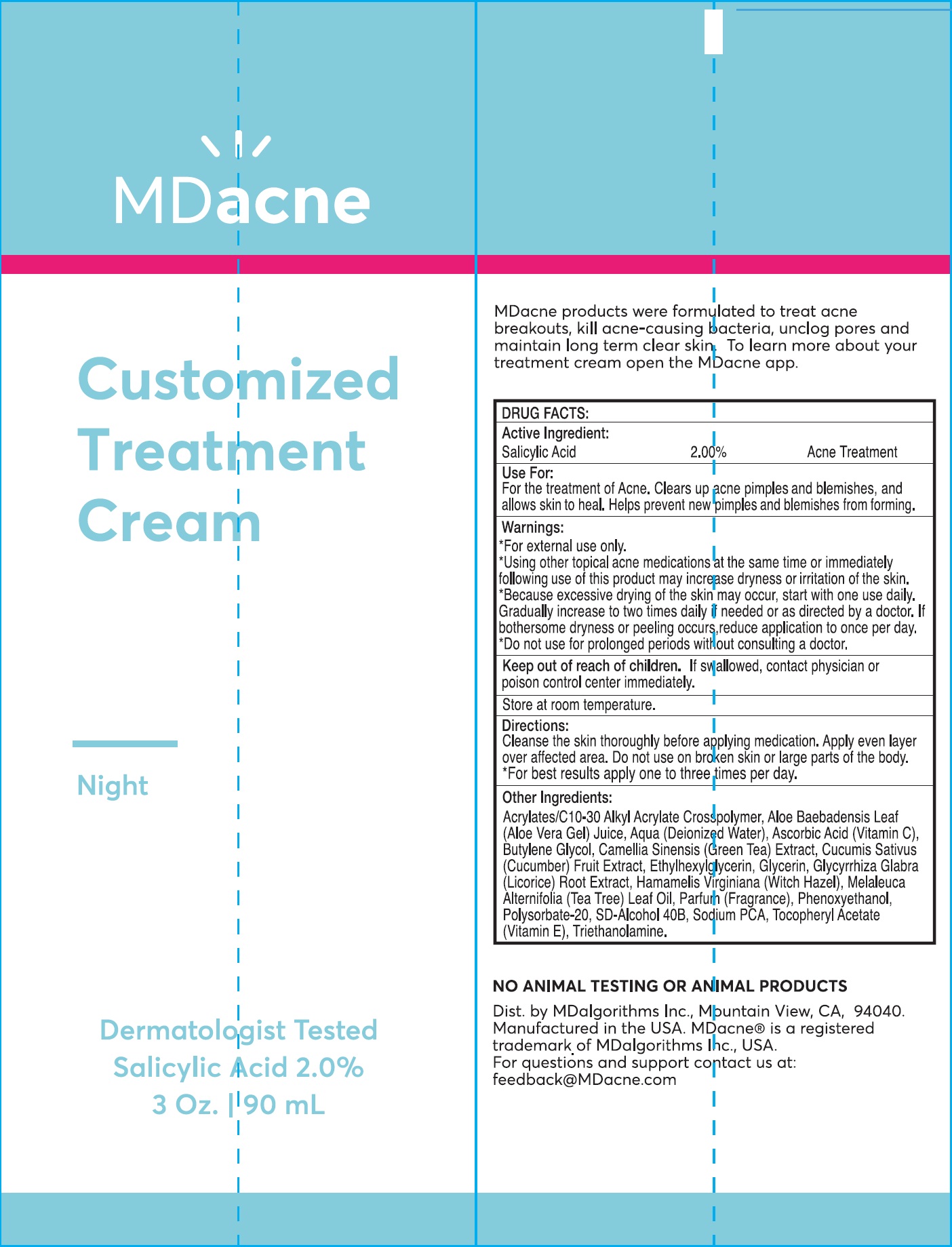
MD ACNE CUSTOMIZED TREATMENT- salicylic acid cream
MDalgorithms Inc
----------
MD ACNE Customized Treatment Cream
Use For:
For the treatment of Acne. Clears up acne pimples and blemishes, and allows skin to heal. Helps prevent new pimples and blemishes from forming.
Warnings:
- For external use only.
- Using other topical acne medications the same time or immerdiately following use of this product may increase dryness or irritation of the skin.
- Because excessive drying of the skin may occur, start with one daily. Gradually increase to two times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once per day.
Directions:
Cleanse the skin thoroughly before applying medication.Apply even layer over affected area. Do not use on broken skin or large parts of the body.
- For best results apply one to three times per day.
Other Ingredients:
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Baebadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Ascorbic Acid (Vitamin C), Butylene Glycol, Camellia Sinensis (Green Tea) Extract, Cucumis Sativus (Cucumber) Fruit Extract, Ethylhexylglycerin, Glycerin, Glycyrrhiza Glabra (Licorice) Root Extract, Hamamelis Virginiana (witch Hazel), Melaleuca Alternifolia (Tea Tree) Leaf Oil, Parfum (Fragrance), Phenoxyethanol, Polysorbate-20, SD-Alcohol 40B, Sodium PCA, Tocopheryl Acetate (Vitamin E), Triethanolamine.
| MD ACNE CUSTOMIZED TREATMENT
salicylic acid cream |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - MDalgorithms Inc (080479826) |