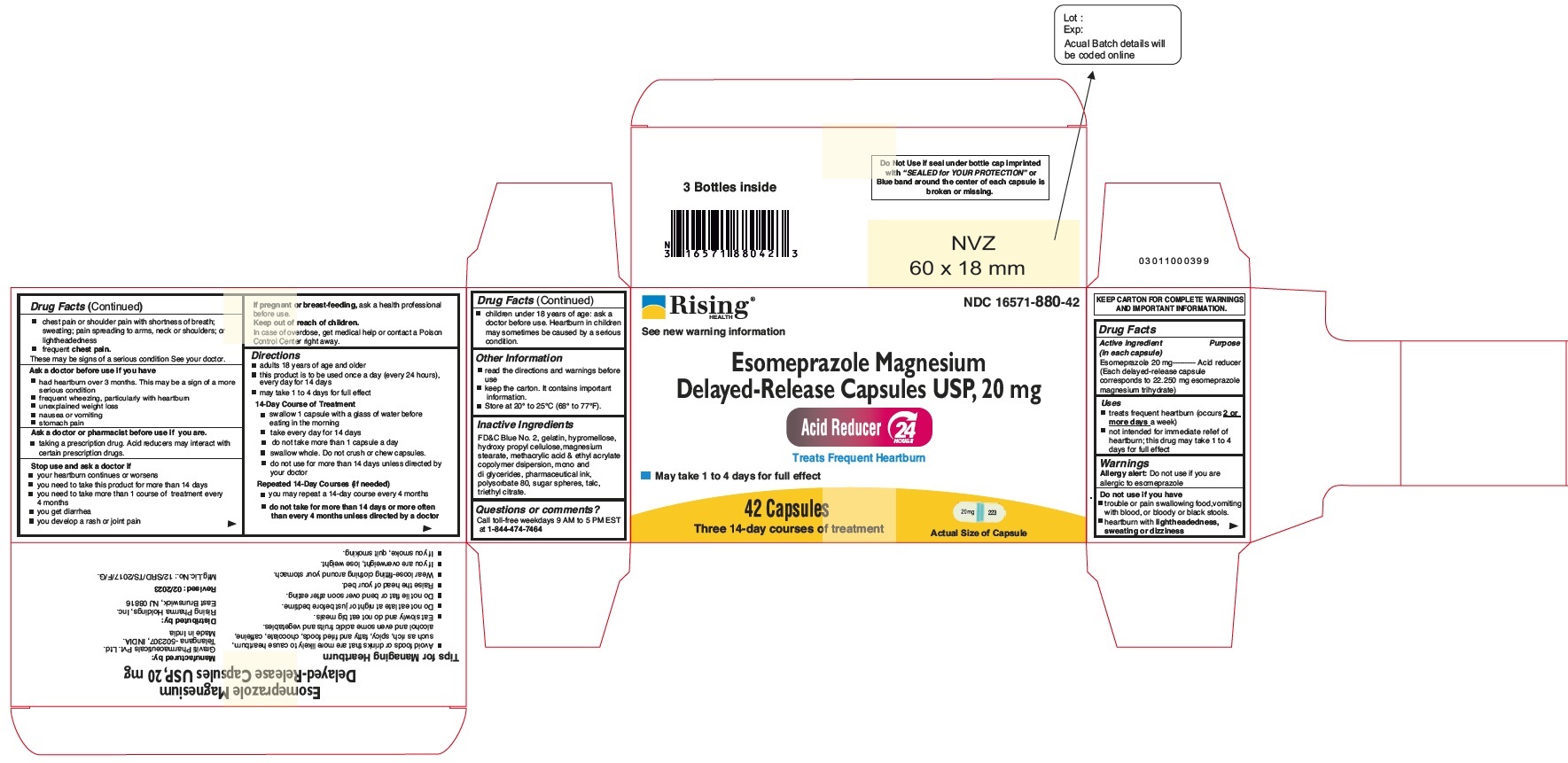
| Active ingredient (in each capsule)
| Purpose
|
| Esomeprazole 20 mg | Acid reducer |
| (Each delayed-release capsule corresponds to 22.250 mg esomeprazole magnesium trihydrate) |
|
- treats frequent heartburn (occurs 2 or more days a week)
- not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
Allergy alert
Do not use if you are allergic to esomeprazole
Do not use if you have
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- you need to take more than 1 course of treatment every 4 months
- you get diarrhea
- you develop a rash or joint pain
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- read the directions and warnings before use
- keep the carton. It contains important information.
- Store at 20° to 25°C (68° to 77°F).
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg:
FD&C Blue 2, Gelatin, Hydroxypropyl Cellulose, Hypromellose, Magnesium Stearate, Methacrylic Acid and Ethyl Acrylate Copolymer Dispersion, Mono-and Di-Glycerides, Pharmaceutical Ink, Polysorbate 80, Sugar Spheres, Talc, Triethyl Citrate.
Questions or comments?
Call toll-free weekdays 9 AM to 5 PM EST at 1-844-474-7464.
Contact Graviti Pharmaceuticals Inc., or www.gravitipharma.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Made in India
See new warning information
- Treats Frequent Heartburn
Esomeprazole magnesium delayed-release capsules 20 mg/acid reducer
May take 1 to 4 days for full effect
14 CAPSULES
One 14-day course of treatment Capsules
Tips for Managing Heartburn
- Avoid foods or drinks that are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some acidic fruits and vegetables.
- Eat slowly and do not eat big meals.
- Do not eat late at night or just before bedtime.
- Do not lie flat or bend over soon after eating.
- Raise the head of your bed.
- Wear loose-fitting clothing
- around your stomach.
- If you are overweight, lose weight.
- If you smoke, quit smoking
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 20 mg Capsules: Container Label
NDC 16571-880-41
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
24 HR
Acid Reducer
Treats Frequent Heartburn
May take 1 to 4 Days for Full effect
14 Capsules
One 14-day Course of Treatment
Distributed by: Rising Pharma Holdings, Inc.

Principal Display Panel - 20 mg Capsules: Carton Label
NDC 16571-880-41
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
24 HR
Acid Reducer
Treats Frequent Heartburn
May take 1 to 4 Days for Full effect
14 Capsules
One 14-day Course of Treatment
Distributed by: Rising Pharma Holdings, Inc.
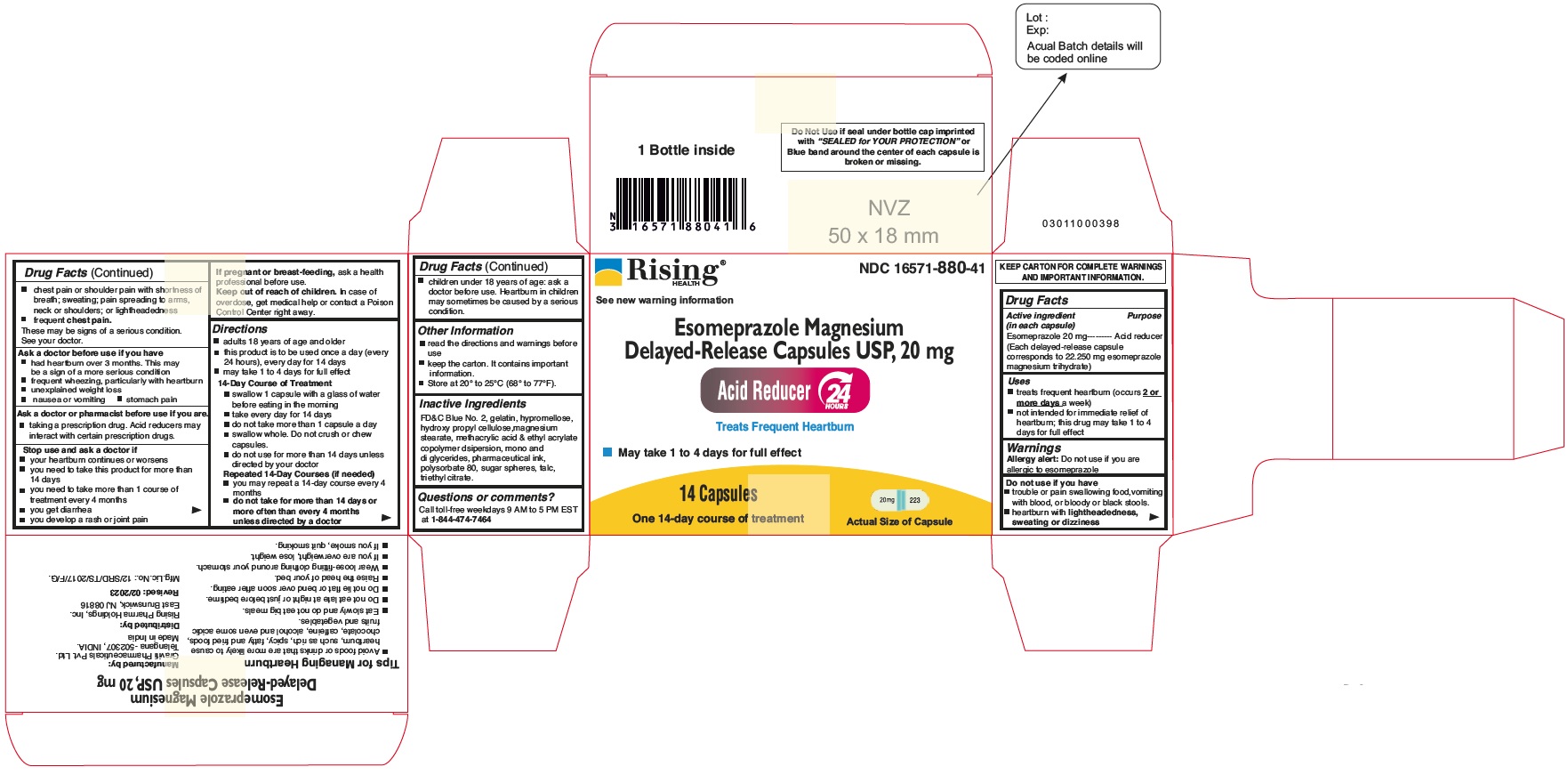
Principal Display Panel - 20 mg Capsules: Carton Label
NDC 16571-880-42
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
24 HR
Acid Reducer
Treats Frequent Heartburn
May take 1 to 4 Days for Full effect
42 Capsules
Three 14-day Courses of Treatment
Distributed by: Rising Pharma Holdings, Inc.