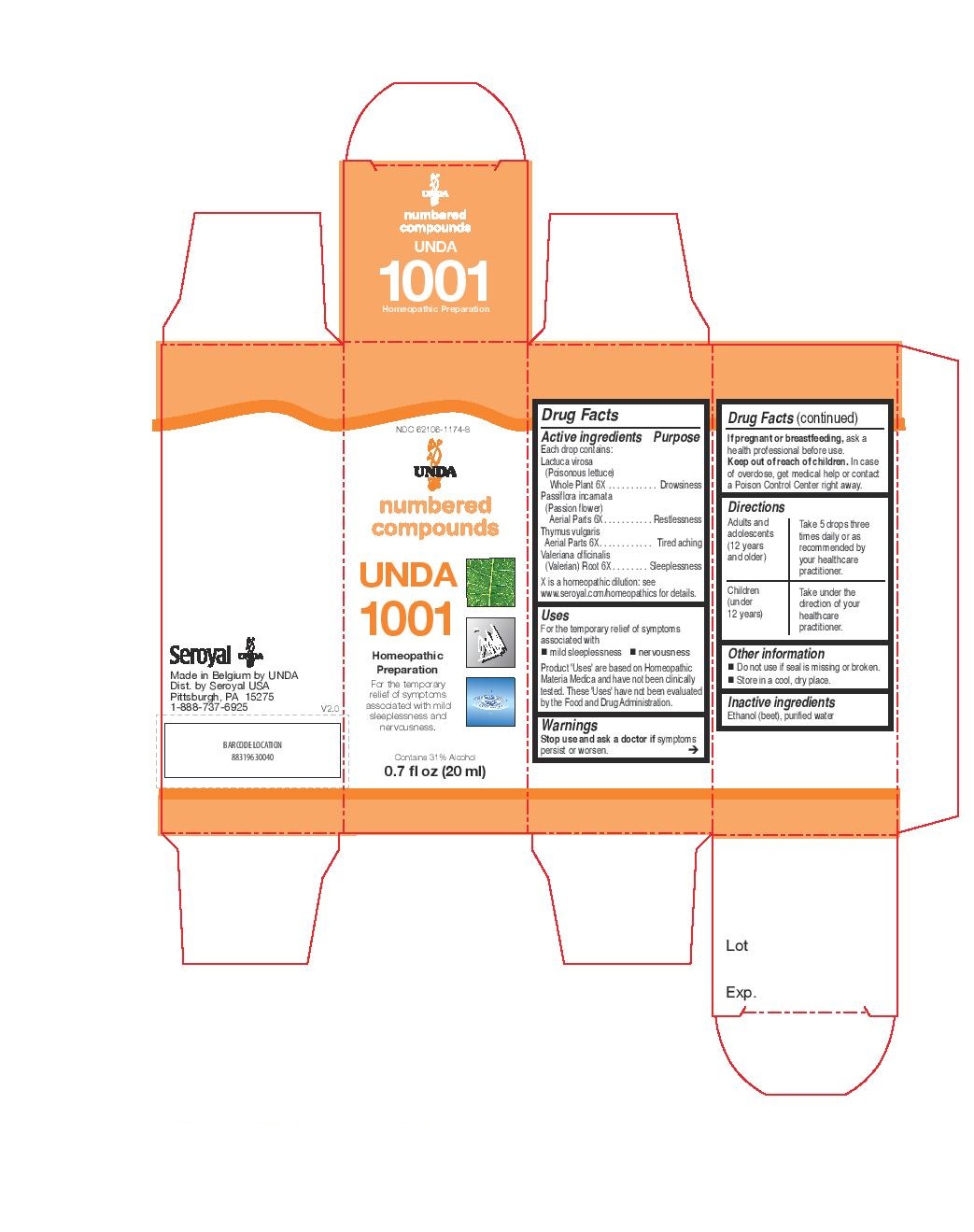
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Asparagus officinalis (Asparagus) Shoot 4X
Berberis vulgaris (Barberry) Bark 4X
Genista tinctoria Aerial Parts 4X
Juniperus communis (Common juniper) Berry 4X
Petroselinum sativum (Parsley) Whole Plant 4X
Rhamnus frangula (Buckthorn) Bark 4X
Thlaspi bursa-pastoris (Shepherd’s purse) Aerial Parts 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with minor fever.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Carum carvi (Caraway) Fruit 4X
Foeniculum vulgare (Fennel) Fruit 4X
Illicium verum (Star anise) Fruit 4X
Rhamnus cathartica (Purging buckthorn) Fruit 4X
Rhamnus frangula (Alder buckthorn) Bark 4X
Senna Leaflet 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with occasional constipation and flatulence.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Active ingredients
Each drop contains:
Aurum metallicum (Gold) 12X
Melissa officinalis (Lemon balm) Leaf and Shoot 4X
Mentha piperita Aerial Parts 4X
Plumbum metallicum (Lead) 12X
Salvia pratensis Leaf 4X
Thymus vulgaris Aerial Parts 4X
Valeriana officinalis (Valerian) Root 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Other information
Do not use if seal is missing or broken.
Store in a cool, dry place.
Contents may not fill package in order to accommodate required labelling. Please rely on stated quantity.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with minor stress and mental fatigue.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Active ingredients
Each drop contains:
Aloe Inspissated juice 6X
Nux vomica (Poison nut) Seed 4X
(2.7 x 10-4 % alkaloids, calculated)
Pulsatilla (Wind Flower) Whole Plant 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with mild indigestion.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Active ingredients
Each drop contains:
Lactuca virosa
(Poisonous lettuce) Whole Plant 6X
Passiflora incarnata (Passion flower) Aerial Parts 6X
Thymus vulgaris Aerial Parts 6X
Valeriana officinalis (Valerian) Root 6X
Warnings
Stop use and ask a doctor if symptoms
persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms
associated with mild sleeplessness and nervousness.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
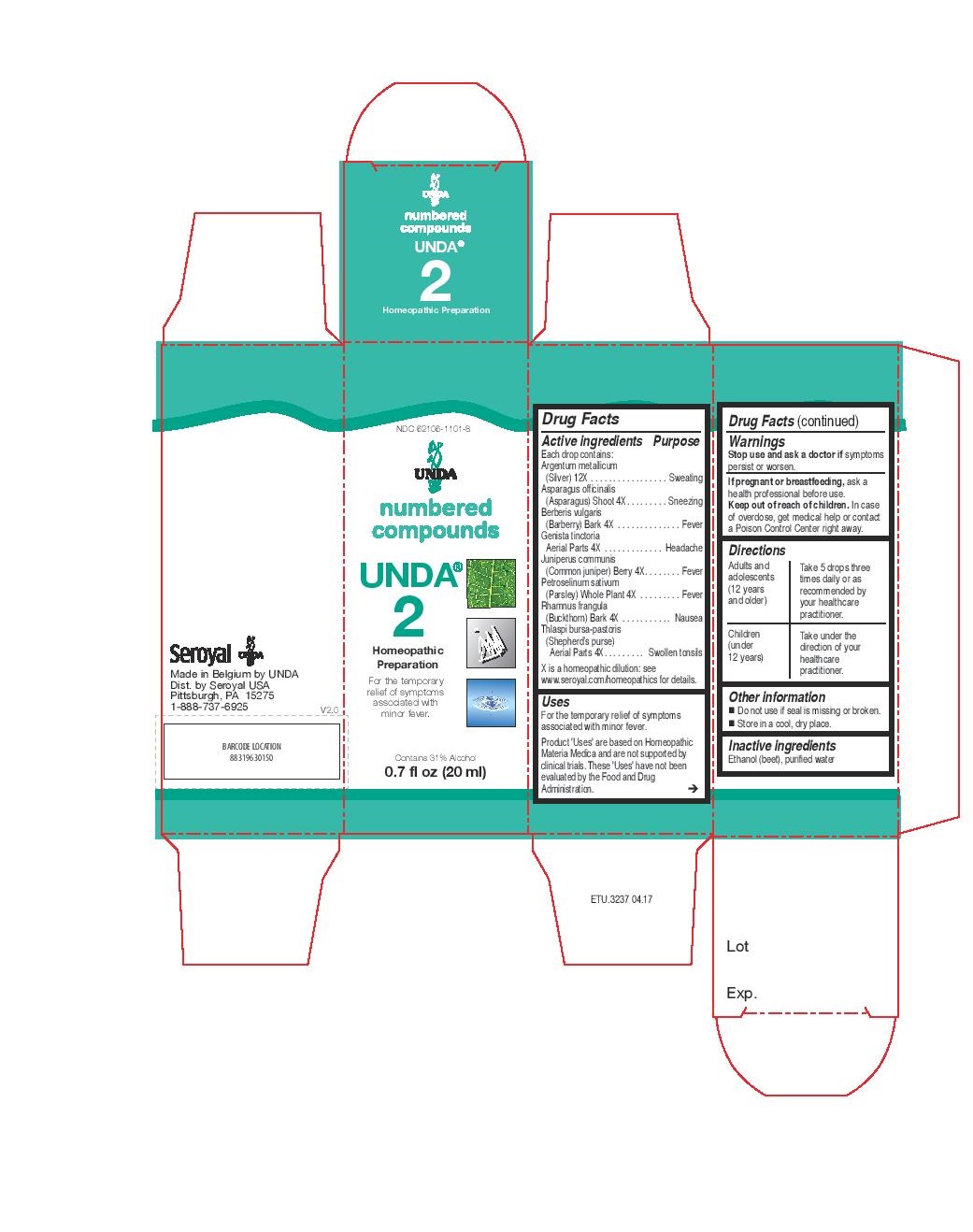
NDC 62106-1101-8
UNDA
numbered compounds
UNDA 2
Homeopathic Preparation
For the temporary relief of symptoms
associated with minor fever.
Contains 31% Alcohol
0.7 fl oz (20 ml)
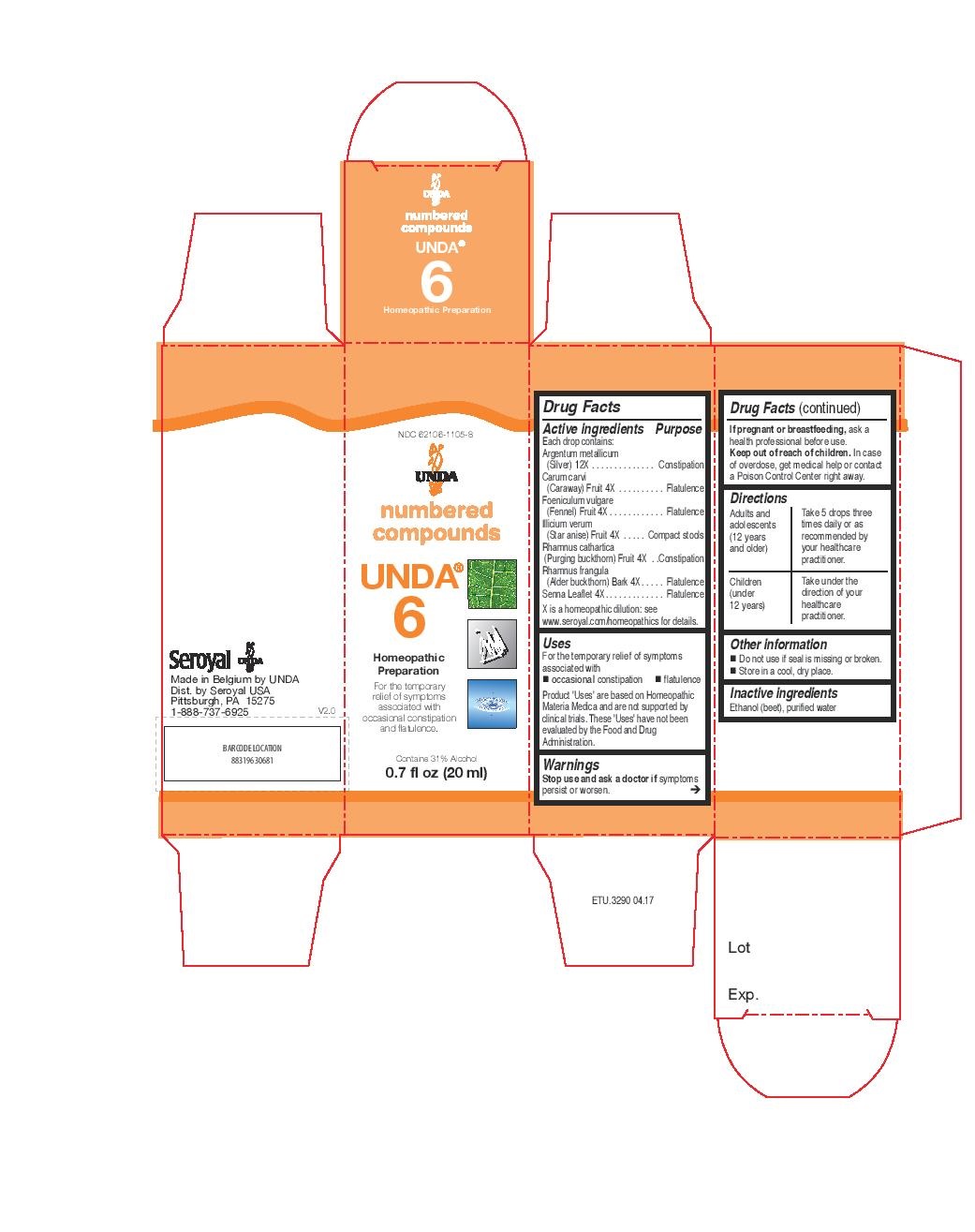
NDC 62106-1105-8
UNDA
numbered compounds
UNDA 6
Homeopathic Preparation
For the temporary relief of symptomsassociated
with occasional constipation and flatulence.
Contains 31% Alcohol
0.7 fl oz (20 ml)

NDC 62106-1108-8
UNDA
numbered compounds
UNDA 9
Homeopathic Preparation
For the temporary relief of symptoms associated
with minor stress and mental fatigue.
Contains 31% Alcohol
0.7 fl oz (20 ml)