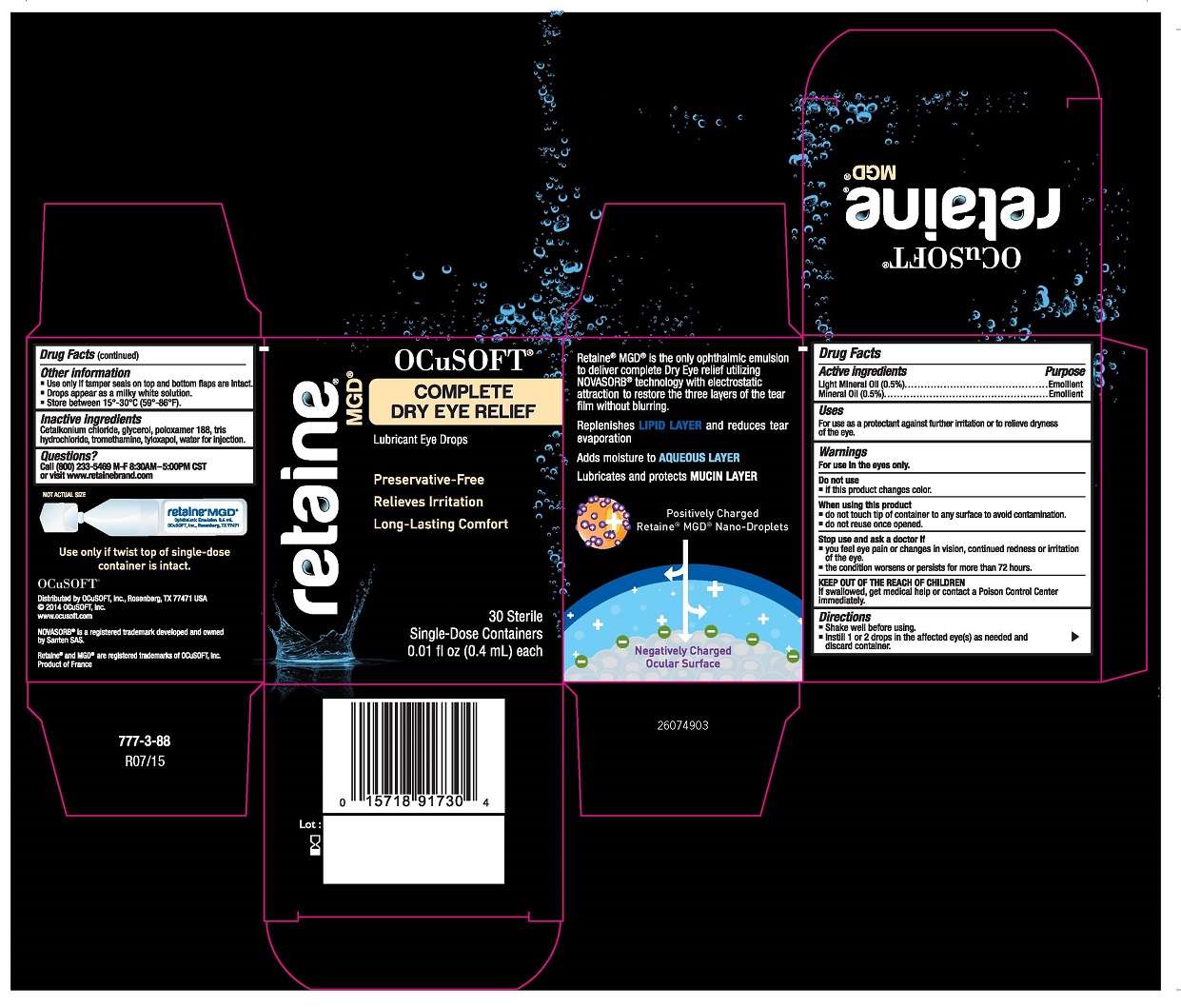
Active Ingredients
Light Mineral Oil (0.5)................................Emollient
Mineral Oil (0.5%).....................................Emollient
When using this product
- do not touch tip of container to any surface to avoid contamination.
- do not reuse once opened.
Stop use and ask a doctor if
- you feel eye pain or changes in vision, continued redness or irritation of the eye.
- the condition worsens or persists for more than 72 hours.
KEEP OUT OF THE REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Shake well before using.
- Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
Other Information
- Use only if printed tamper seals on top and bottom flaps are intact.
- Drops appear as a milky white solution.
- Store between 15 o-30 oC (59 o-86 oF).