Uses
- Temporarily relieves minor aches and pains due to :
- headache
- muscular aches
- backache
- minor pain of arthritis
- the common cold
- toothache
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 10 softgels (3,250 mg) in 24 hours for adults or 5 softgels (1,625 mg) in 24 hours for children. Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
Do not take more than directed
(see overdose warning)
|
Adults and children 12 years and over |
|
|
Children 6-11 years |
|
|
children under 6 years |
|
Inactive ingredients
FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol special and white edible ink.
Manufactured by:
Humanwell PuraCap Pharmaceutical (Wuhan) Ltd.
Wuhan, Hubei
430206, China
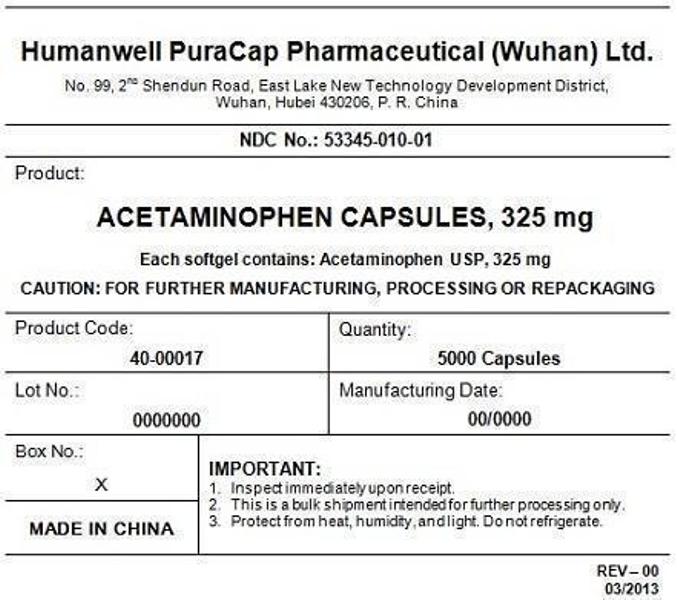
PRINCIPAL DISPLAY PANEL - Shipping Label
ACETAMINOPHEN CAPSULES, 325 mg
Quantity : 5000 Capsules
NDC. No : 53345-010-01
IMPORTANT:
Inspect immediate upon receipt.
This is a bulk shipment intended for further processing only.
Protect from heat, humidity, and light. Do not refrigerate.
CAUTION : "FOR FURTHER MANUFACTURING, PROCESSING OR REPACKING"